
New York City Department of Education
Institutional Review Board
Policy Guide
Created August 2023
Updated May 2024
Research and Policy Support Group
Office of Policy and Evaluation
2
Table of Contents
Contents
1. Overview ............................................................................................................................................... 5
1.1 Purpose of this document ............................................................................................................. 5
1.2 Research Oversight Jurisdiction .................................................................................................... 5
1.2.1 NYC DOE Institutional Review Board -- Updated May 2024 ................................................. 5
1.2.2 Do I need to submit to the NYC DOE IRB? -- Updated May 2024 ......................................... 6
1.3 Legal and Policy Regulations ......................................................................................................... 8
2. The NYC DOE Institutional Review Board (IRB) ..................................................................................... 9
2.1 Research and Research Ethics -- Updated May 2024 ................................................................... 9
2.2 The Board .................................................................................................................................... 10
3. Types of Research and Reviews .......................................................................................................... 11
3.1 Engagement in research ............................................................................................................. 11
3.2 Types of Studies .......................................................................................................................... 14
3.2.1 Internal research ................................................................................................................. 14
3.2.2 Collaborative research -- Updated May 2024 ..................................................................... 15
3.2.3 External research ................................................................................................................ 15
3.2.4 Administrative Data Analysis .............................................................................................. 17
3.2.5 Activities that are not technically Human Subjects Research ............................................. 17
3.2.6 Research conducted by NYC DOE students -- Updated May 2024 ..................................... 18
3.3 Types of Review .......................................................................................................................... 18
3.3.1 NYC DOE IRB Review ........................................................................................................... 18
3.3.2 NYC DOE Research Policy Review ....................................................................................... 19
4. The NYC DOE IRB Process.................................................................................................................... 21
4.1 Process Graphic ........................................................................................................................... 21
4.2 Review Process Steps .................................................................................................................. 21
4.3 Using IRB Manager ...................................................................................................................... 22
4.3.1 Before you begin: ................................................................................................................ 22
4.3.2 To create an account:.......................................................................................................... 22
4.3.3 To submit an IRB protocol or Data Request: ....................................................................... 22
4.4 Submitting to IRB Manager ......................................................................................................... 23
4.4.1 General Instructions ............................................................................................................ 23
4.4.2 Protocol xForm .................................................................................................................... 24
3
4.4.3 Credentialing ....................................................................................................................... 25
4.4.4 District Sponsor Request Form ........................................................................................... 25
4.4.5 Data Request ....................................................................................................................... 25
4.4.6 Amendment -- Updated May 2024 ..................................................................................... 25
4.4.7 Protocol Violation/Deviation .............................................................................................. 26
4.4.8 Continuation -- Updated May 2024 .................................................................................... 26
4.4.9 Closure ................................................................................................................................ 27
4.4.10 Other processes .................................................................................................................. 27
5. NYC DOE Research Policies ................................................................................................................. 28
5.1 Considering doing research in NYC public schools ..................................................................... 28
5.1.1 Demonstrating value to the DOE -- Updated May 2024 ..................................................... 28
5.1.2 Burden ................................................................................................................................. 30
5.1.3 Equity .................................................................................................................................. 30
5.1.4 In-person or Virtual Studies -- Updated May 2024 ............................................................. 31
5.1.5 Study Personnel -- Updated May 2024 ............................................................................... 31
5.1.6 Research Design -- Updated May 2024 ............................................................................... 36
5.1.7 FERPA & PPRA ..................................................................................................................... 40
5.1.8 Data Security ....................................................................................................................... 41
5.2 Recruitment and Consent ........................................................................................................... 41
5.2.1 Research site inclusion/exclusion criteria ........................................................................... 41
5.2.2 Principal Letter -- Updated May 2024 ................................................................................. 41
5.2.3 Participant Recruitment -- Updated May 2024 .................................................................. 42
5.2.4 Participant Screening -- Updated May 2024 ....................................................................... 45
5.2.5 Participant Consent -- Updated May 2024 ......................................................................... 46
5.3 Interactions with Research Subjects ........................................................................................... 51
5.3.1 Asking children sensitive questions .................................................................................... 51
5.3.2 Procedures for conducting school or classroom observations -- Updated May 2024 ........ 51
5.3.3 Conducting Focus Groups -- Updated May 2024 ................................................................ 53
5.3.4 Conducting Interviews -- Updated May 2024 ..................................................................... 53
5.3.5 Collecting artifacts .............................................................................................................. 54
5.3.6 Collecting data from educational applications -- Updated May 2024 ................................ 54
5.3.7 Collecting any medical or biometric data ........................................................................... 54
5.3.8 Recording (audio, video, photography) .............................................................................. 54

4
5.3.9 Compensation for research -- Updated May 2024 ............................................................. 56
5.3.10 Deception or Non-Disclosure -- Updated May 2024 ........................................................... 58
6. Appendices .......................................................................................................................................... 60
6.1 Definitions ................................................................................................................................... 60
6.2 CITI Training Requirements ......................................................................................................... 60
6.3 Guidance for DOE students completing research -- Updated May 2024 ................................... 62

5
1. Overview
1.1 Purpose of this document
This Policy Guide documents policies governing research and evaluation in the New York City
Department of Education (NYC DOE) in order to support research facilitation and oversight.
This guide:
• Clarifies the policies in place to protect the rights of students, staff, and families.
• Makes doing research in NYC public schools more accessible to researchers by providing explicit
guidance on processes, requirements, and policies.
• Promotes transparency by describing internal administrative processes, documenting DOE
research policies and requirements, and explaining considerations in the review process.
• Intends to make research in NYC public schools more relevant and beneficial to our students by
explaining policies prioritizing research that brings direct value to the NYC DOE.
If you have any questions after reading this guide, please reach out to [email protected].
1.2 Research Oversight Jurisdiction
The New York City Department of Education’s Research Policy and Support Group (RPSG) oversees and
facilitates human subjects research (HSR), non-HSR evaluation, and the use of administrative data for
research and/or evaluation through the NYC DOE Institutional Review Board and the Data Request
Committee.
1.2.1 NYC DOE Institutional Review Board -- Updated May 2024
In 1980, the New York City Department of Education established the Proposal Review Committee, now
known as the Institutional Review Board (NYC DOE IRB), to review all requests to conduct data collection
for research and evaluation in NYC public schools. Any studies being conducted in schools or with NYC
public school students, staff, or affiliates must be reviewed and approved by the NYC DOE IRB to ensure
they comply with DOE policies, protect the privacy of students, parents, and staff, and do not disrupt the
work of students and staff.
The NYC DOE IRB must review and approve any studies within its jurisdiction. This jurisdiction includes,
but is not limited to:
• Any study conducted by an external person/organization with NYC DOE students, families,
school staff, non-school based staff, or staff of organizations contracted by the DOE (including
CBOs, such as PreK providers)
• Any study conducted in a NYC DOE school or other physical or virtual site where DOE affiliates
are involved
• Any study (including program evaluations or studies deemed not human subjects research) that
involves students, is conducted by an external organization, and was not initiated on the DOE’s
behalf
• Any study that involves access to individual-level NYC DOE administrative data (students, staff,
families) or aggregate-level administrative data not publicly available

6
• Any NYC DOE employee of office engaged in human subjects research as part of their
employment
• Other research and evaluation work, as determined by the NYC DOE IRB
Many internal program evaluation, continuous improvement, and other activities occur in NYC public
schools that are not technically considered human subjects research (HSR). However, in some cases the
NYC DOE IRB may conduct a DOE Research Policy Review for non-HSR evaluations if certain conditions
exist (including, a sensitive participant population, multi-agency involvement, data sharing outside of
the DOE, involvement of students, involvement of an external party, or if the project was not initiated
on the DOE’s behalf). Please reach out to the IRB inbox (IRB@schools.nyc.gov) if you have questions
about if your study needs to be reviewed by the IRB.
Research with charter and private schools typically does not fall within the jurisdiction of the NYC DOE
IRB. However, if charter or private schools are also part of a study that includes traditional public
schools, this should be stated in the IRB protocol submission.
Oversight role
• The NYC DOE IRB typically serves as the IRB of Record for studies conducted by NYC DOE
employees conducting research as part of their official work responsibilities.
• The NYC DOE IRB may conduct a DOE Research Policy Review for collaborative or external
studies approved by an external IRB but conducted with NYC DOE affiliates or in NYC DOE
schools.
• Depending on the type of study and the extent to which DOE affiliates are engaged in the study,
the DOE IRB may conduct different levels of review. See Types of Review for more information.
1.2.2 Do I need to submit to the NYC DOE IRB? -- Updated May 2024
Review the following table to determine if your study needs to be reviewed and approved by the NYC
DOE IRB.
DOE employees or affiliates who are conducting research to complete a graduate degree are
considered external researchers and must receive approval from their institution’s IRB before
submitting to the DOE IRB. Even though the researcher is a DOE employee, they are not conducting
their dissertation research on behalf of the DOE, so the study is considered external. Additionally,
the DOE employee must still receive a determination from the Conflicts of Interest Board to conduct
research in DOE schools (see here for more info on the COIB requirements).

7
Do I need to submit my study to the NYC DOE IRB?
Must submit
Do not need to submit
Research
A research study, including but not limited to
human subjects research, conducted by an
external party in which activities (e.g.
recruitment, screening, consent, data collection)
occur in NYC DOE schools or virtual spaces, or in
which potential subjects are included because of
their status as a NYC DOE student, staff member,
or family.
A research study in which no component,
including but not limited to recruitment,
consent, and data collection, occurs in NYC
DOE schools or virtual spaces (e.g. Google
Classroom) AND potential subjects'
likelihood of inclusion in the study is in no
way related to their status as a NYC DOE
student, staff, or family.
Any NYC DOE employee or office engaged in
human subjects research as part of their DOE job
responsibilities.
Any NYC DOE staff conducting research, including
but not limited to human subjects research,
within RFOC’s jurisdiction but outside of their
official NYC DOE responsibilities, including staff
who are completing a dissertation or other type
of project for a higher education degree.
Other research, as determined by the RFOC.
Evaluation & Program Improvement
Program evaluations or data collection for
program improvement, conducted by external
organizations.
Program evaluations or data collection for
program improvement, conducted
internally (by a NYC DOE program team,
office, or school), as part of individuals’ or
teams’ DOE official responsibilities.
Any NYC DOE staff conducting program
evaluation within RFOC’s jurisdiction but outside
of their official NYC DOE responsibilities, including
staff who are completing a dissertation or other
type of project for a higher education degree.
Programmatic data collected for a program, and
used for any purpose aside from directly serving
current program participants. For example, data
collected for programmatic implementation that
you want to use to evaluate the program.
Programmatic data collected solely for the
purpose of directly serving the recipients of
a program (such as, collecting student
assessments to determine the level of math
intervention they should receive).
Other evaluation work, as determined by the
RFOC.

8
Additional questions to consider:
• Has this project been reviewed by an external IRB already? If so, what was the determination?
o If an external IRB reviewed your study as any category of minimal risk, Expedited,
Exempt, or Full Board, you will need to submit to the NYC DOE IRB.
o If an external IRB reviewed your study as “not research” or some other not applicable
category, please email the IRB inbox ([email protected].gov) to determine if you need to
submit.
• What data is being collected?
o If you are collecting data from DOE staff about their thoughts, feelings, or opinions, you
will likely have to submit to the NYC DOE IRB.
o If you are collecting information from DOE central office staff about programmatic
operations or job functions, this may not require NYC DOE IRB review.
• Will you be collecting any data from students or families?
o If you are collecting any data from students or families, you will need to submit to the
NYC DOE IRB.
• How will the results or findings be used and/or shared? Who is the intended audience for the
findings? Do you plan to publish any of the findings?
o If results will be published or shared with an external audience (such as academic
journals, conferences, or funders), you will need to submit to the NYC DOE IRB.
Please note, submission to the NYC DOE IRB for review is required even if you have already received
approval from your institutional IRB.
Studies are reviewed on a case-by-case basis, and studies that do not fall into the categories above may
still need to be reviewed by the NYC DOE IRB. Please email [email protected]yc.gov with questions.
1.3 Legal and Policy Regulations
[Coming soon.]

9
2. The NYC DOE Institutional Review Board (IRB)
2.1 Research and Research Ethics -- Updated May 2024
The NYC DOE IRB aligns with the basic ethical principles and guidelines for the protection of human
subjects in research, defined in the Belmont Report.
Respect for Persons
- Respect the autonomy of individuals
o Respect personal decisions, and provide necessary information to make decisions
o Subjects enter into research voluntarily, and with adequate information
- Protect individuals with diminished autonomy (such as, children)
o Ensure individuals take part in activities voluntarily, and with awareness of possible
adverse consequences
o Exclude individuals from activities that may harm them
o Depends on the risk of harm and the likelihood of benefit
Beneficence
- The obligation to do no harm
- The obligation to maximize possible benefits and minimize possible harms
Justice
- Consider who bears the burden of research, and who benefits from the research
- Consider if some populations are systematically selected for research because of their easy
availability, compromised position, or manipulability
The NYC DOE IRB follows the definitions as described in the Basic HHS Policy for Protection of Human
Research Subjects (link), including:
• Human subject: A living individual about whom an investigator (whether professional or
student) conducting research:
o (i) Obtains information or biospecimens through intervention or interaction with the
individual, and uses, studies, or analyzes the information or biospecimens; or
o (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or
identifiable biospecimens.
• Intervention: Includes both physical procedures by which information or biospecimens are
gathered (e.g., venipuncture) and manipulations of the subject or the subject’s environment
that are performed for research purposes.
• Interaction: Includes communication or interpersonal contact between investigator and subject.
• Private information: Includes information about behavior that occurs in a context in which an
individual can reasonably expect that no observation or recording is taking place, and
information that has been provided for specific purposes by an individual and that the individual
can reasonably expect will not be made public (e.g., a medical record).
• Identifiable private information: Private information for which the identity of the subject is or
may readily be ascertained by the investigator or associated with the information.
• Research: A systematic investigation, including research development, testing, and evaluation,
designed to develop or contribute to generalizable knowledge.

10
2.2 The Board
[Coming soon.]
IRB Registration: The NYC DOE IRB is registered with the United States (US) Department of Health and Human Services
(DHHS) Office for Human Research Protections (OHRP). The DOE IRB registration number is: IRB00011754 and
IRB00012206.
Federal Wide Assurance: The DOE has filed a Federal Wide Assurance (FWA) with the US DHHS OHRP, which documents
the DOE’s commitment to comply with federal regulations for the protection of human subjects in research. The DOE FWA
number is as follows: FWA00005811.

11
3. Types of Research and Reviews
This section details the different types of research and analysis that may be conducted in NYC public
schools, and how proposals to conduct these types of research may be reviewed. However, before
considering the different types of studies and review, it is important to understand how the DOE is
engaged or not engaged in the study.
3.1 Engagement in research
To adhere to federal requirements for research oversight, and to determine what institution’s IRB
should serve as the IRB of Record for a research study, it is important to understand what entities are
“engaged in research.” Understanding this also helps determine if a study should be internal,
collaborative, or external.
Federal guidance released in 2022 [link] recommends the following definition:
A party is “engaged in research” if it (or its employees, staff, or agents) has a key role in
designing the research, conducting the research, analyzing and interpreting the results, or
gaining informed consent from human subjects.
To align with this guidance, the NYC DOE IRB defines engagement in research in the following way.
Defining “engaged in research”
The following definitions are a guide for how the NYC DOE IRB may consider engagement in research.
However, every protocol is reviewed on a case-by-case basis.
The NYC DOE IRB distinguishes between “engaged” and “not engaged” in research by who has
operational and/or intellectual control over the research, including who is designing the study, and
who has the power to make decisions and judgement calls in the research implementation.
Someone would likely be considered “engaged in research” if they:
• Design any part of the research
• Recruit research participants, or track research subject participation (e.g., track consent form
completion, follow up with potential participants)
• Conduct the research (e.g., conduct interviews or focus groups)
• Access or analyze individual-level or identifiable data collected through research activities
• Obtain informed consent from human subjects (e.g., explain the study, answer questions)
• Engage in interpretation of results, writing findings, or are cited as an author on any publication
Someone would likely be considered “not engaged” in research if they only:
• Provide feedback on research design, survey questions, etc.
• Provide relevant contextual information about NYC DOE operations
• Do not have access to any data collected as part of the research, but may advise on
methodology (e.g., a methods advisor)

12
• Provide access to a research activity (e.g., put parent consent forms in students’ backpacks,
passively forward recruitment emails to school staff over whom they have no supervisory
authority)
• Collect signed informed consent materials or provide factual information to potential
participants (e.g., collect signed forms in a box to give to researchers, or direct participants to
the researchers to answer questions about the study)
What if a DOE employee is “engaged in research”?
In cases where a DOE employee is engaged in research, they must be listed on the protocol as part of
the research team, must obtain CITI certification, and their activities must be detailed in the protocol. If
the study is being done by an external researcher and a DOE employee together, the study would likely
be identified as collaborative, depending on the degree of engagement by the external researchers and
the DOE employee. The IRB of Record and appropriate PI would be determined by the level of
engagement of each person/institution engaged in the research. See the Types of Studies section for
more info on collaborative, external, and internal studies.
Another option: The DOE Advisor
Sometimes a DOE employee works closely with the research team to provide feedback on the study and
share information about DOE priorities. However, the DOE employee does not have operational or
intellectual control of the research; they are not engaged in the research. In these cases, the DOE
employee may be listed on the protocol as a “DOE Advisor” as part of the research team. Depending on
their role, the DOE Advisor may not need CITI certification and the study may not need to be listed as
collaborative. However, the DOE Advisor’s involvement must be detailed in the protocol. The purpose of
listing the DOE employee and describing their role is so the DOE IRB knows how any DOE employees are
involved in the study (even if they are not formally “engaged in research”), and so the Board can make
more informed decisions about how the study may benefit the DOE.
[Please note, the current application xform available in IRB Manager does not yet have a formal field for
the DOE Advisor. Please instead include in the narrative description of the project.]
The ”school coordinator”
[Coming soon.]
If the DOE is a Federal Funding Awardee
When the DOE is the primary awardee for federal research funding, this does not mean the DOE is
automatically “engaged,” since the DOE may contract research work out to sub-awardees. Federal
guidance recommends determining “engagement” in research by who has control and decision-making
power over the research. If the DOE serves as only a pass-through entity for funding, but has no
operational and/or intellectual control over the research, they may not be considered “engaged.”

13
Examples of the DOE being “engaged” or “not engaged” in research
If a DOE affiliate does any of the following
activities, they would be considered:
ENGAGED
If a DOE affiliate only does the following
activities, they would be considered:
NOT ENGAGED
Authorship
Would be an author or co-author on the study in
their DOE capacity
Is included in a paper’s
acknowledgements
Study
design
Co-write research questions
Share feedback on what research
questions and participant populations
are of most interest to the DOE
Instrument
design
Write survey protocol
Provide edits to question wording and
technical terms that are particular to the
DOE, to ensure understandability
Recruitment
Track student rosters to see who has returned
consent forms and follow up with parents
Select a random or representative sample of
participants
Recruitment procedures:
• Use DOE channels (such as Principals Digest)
• State that the DOE endorses the study
• List a DOE affiliate on recruitment materials
Brainstorm ways to improve
participation response rates
Provide rosters to researchers through a
FERPA exception
Passively forward an email about the
research to people they do not
supervise
Consenting
Answer participant questions about the consent
form
Pass out consent forms for students to
backpack home
Collect completed consent forms for
researchers to retrieve
Direct participants to the researchers to
answer any questions
Data
collection
Administer a 1-on-1 assessment with a child for
research, outside of normal job responsibilities
Access identifiable data collected through the
research (e.g., a teacher inputs paper survey
data into a database for researchers)
Monitor and respond to subject distress
Administer a 1-on-1 assessment on a
child as part of normal job
responsibilities
Collect completed paper surveys from
parents who filled it out at home
Data
analysis
Access data collected through research to advise
on analysis methods, or conduct any data
analysis
Advise on methods only, and do not
access any research data
Sharing
findings
Write, revise, or substantively change the
meaning of findings that are included in any
publications
Coordinate logistics and invitations for a
presentation where researchers share
findings with a school community

14
3.2 Types of Studies
After determining who is engaged in research, and to what extent they are engaged, you can determine
what kind of study you plan to conduct. Studies may be Internal, Collaborative, or External. Determining
the type of study relies on the NYC DOE IRB’s definition of “engaged in research,” and aligns with federal
guidance on cooperative research and single IRB requirements.
3.2.1 Internal research
Internal studies are human subjects research conducted by NYC DOE staff or affiliates for the purposes
of the DOE, where the DOE is the institution conducting the research. The NYC DOE IRB will serve as the
IRB of Record for internal studies, and the DOE staff member or affiliate would be listed as the PI.
A study may be internal if:
• A DOE employee is conducting human subjects research as part of their work for the DOE.
• A DOE program team designs a human subjects research project, is the primary decision-maker,
and hires an external research firm to implement a survey. In this case, the DOE affiliates are
likely “engaged in research” and the hired research firm may not be formally “engaged.” The
DOE IRB would use the extent of each party’s engagement to determine if the study is internal.
A study may not be internal if:
• A DOE employee is conducting human subjects research in order to complete a dissertation to
fulfill the requirements of a graduate degree. This would likely be external, and the employee
would be required to receive approval from their university IRB before submitting to the DOE
IRB.
• A DOE program team hires an external research firm and collaborates with them to design and
implement a human subjects research project, where both entities have decision-making power.
This would likely be collaborative. In this case, both the DOE team and the external research
firm are “engaged in research.” The IRB of Record would likely be determined by each party’s
degree of engagement.
The DOE often conducts internal evaluations of program implementation for the purposes of
continuous improvement. These are not considered human subjects research because they are intended
for program improvement, and are not intended to contribute to generalizable knowledge. Since they
are not human subjects research and they are conducted internally to the DOE, these projects typically
do not need to be approved by the NYC DOE IRB. DOE staff conducting internal evaluations may still
request guidance from the NYC DOE IRB, and are encouraged to reach out with questions about
protecting subjects and data.
Please note, external evaluations that are not technically human subjects research may still need to be
reviewed by the NYC DOE IRB. Please see section: “Activities that are not technically Human Subjects
Research” for more information.

15
3.2.2 Collaborative research -- Updated May 2024
Collaborative studies, also known as cooperative or multi-site studies, are projects where more than one
institution is “engaged in the research” and one of those institutions is the NYC DOE [please see above
section on “Engagement” for a definition of engagement in research]. A study by two or more
institutions working together, but not including the NYC DOE, would be considered an “External” study.
Collaborative studies may be composed of:
• A NYC DOE program team and an external research institution working together on a study
• The NYC DOE working with another city agency and a university on a study
• Some other collaboration, including the NYC DOE
IRB of Record
• In collaborative studies, the IRB of Record is typically determined by who is the PI, and how
much each institution is engaged in the research. This is determined on a case-by-case basis.
• The NYC DOE IRB is typically the IRB of Record if:
o A DOE staff member is the PI
o DOE has hired a research organization to implement a research study that the DOE has
already designed
• An external IRB is typically the IRB of Record if:
o The external partner is the PI
o The DOE is the prime awardee of research funding, and the external organization is a
sub-awardee and holds operational and intellectual control over the research
Additional requirements for collaborative studies
• A collaborative study must have at least one DOE staff member listed as a member of the
research team, and they must be CITI trained. The DOE staff member does not need to be the PI.
• Documentation of external review and approval for all collaborating institutions and researchers
is required and must be attached to the protocol once obtained.
• It may be necessary to execute a reliance agreement between the NYC DOE IRB and the external
IRB. Please reach out to IRB@schools.nyc.gov with questions about reliance agreements.
3.2.3 External research
External studies are projects conducted by universities, research institutions, or other organizations.
External studies must be reviewed and approved by an external IRB before they can be submitted to the
NYC DOE IRB. The external IRB would serve as the IRB of Record. In some cases, an external IRB may not
fully approve a study until the DOE IRB has approved. In these cases, the external IRB can review and
provide conditional approval contingent on approval from the NYC DOE IRB.
External studies may include:
• A research firm conducting a study on a curriculum being implemented in NYC schools. In this
case, the external research firm would serve as the IRB of Record.
• A DOE employee who is also a PhD student and is conducting research to complete a
dissertation. In this case, the university where the person is completing their PhD would serve as
the IRB of Record.

16
Requirements for external studies
• Submissions of external research to the NYC DOE IRB must include:
o Documentation of external IRB review and approval
o The protocol application submitted to the external IRB
• The information, study design, and materials submitted to the DOE IRB must align with the
research design and study materials that were approved by the external IRB, or any differences
must be explained.
• For dissertation studies, we recommend that the PhD student serve as the PI. However, there
may be cases where it is more appropriate for the faculty advisor to serve as the PI. This is up to
the researcher to determine, and may depend on who is the primary decision-maker in the
research, or who is bearing the primary responsibility for ensuring the protection of human
subjects in the research. Please note, even if the faculty advisor is the PI, if the PhD student is a
DOE employee, they must obtain a Conflicts of Interest determination.
Review process
• For studies where an external IRB has reviewed and approved as exempt or expedited, the NYC
DOE IRB may conduct an expedited DOE Research Policy Review. However, the DOE IRB may
bring a protocol to the full board for any reason, or if any of the following apply:
o Research involves children
o Protocol requests a waiver of documented informed consent
o Protocol wants to video or audio record children
o Interview, focus group, or survey instruments ask sensitive questions of children
o Protocol wants to collect any medical or biometric data
• Please see below section on Types of Review for more information on how protocols are
reviewed.
DOE involvement in external research
Sometimes DOE program teams support external research. To determine if a study should be listed as
Collaborative or External, consider if the DOE is “engaged in research,” as defined above.
DOE affiliates may be involved with external research and it would not constitute a collaborative study.
This would depend on if the DOE affiliate is considered “engaged in research.” If the DOE affiliate is not
formally engaged based on the DOE definition, the study could likely be external. In this case, it may be
appropriate to list a DOE staff member on the IRB protocol as a “DOE Advisor,” and detail their
involvement. Additionally, the presence of a DOE Advisor would not necessarily require the study to be
collaborative. This is detailed more in the Engagement in Research section. Please note, if the DOE
person is “engaged in research” at all, the study would likely become collaborative.
“Engaged in research” vs Research “on behalf of the DOE”
In some cases, the research will need to submit a Data Request for student-level data, and in order to
qualify for a FERPA “studies” exception to access this data, the project will need a letter of support from
a DOE staff member to confirm the study is being done “on behalf of the DOE.” This letter of support
and statement that the research is “on behalf of the DOE” does not necessarily mean a study is

17
collaborative. A study can still be external even if the DOE is interested in and supports the study. When
a DOE affiliate becomes “engaged” in the research, you will need to consider if the study is
collaborative.
3.2.4 Administrative Data Analysis
[Coming soon.]
See External Data Request FAQs.
3.2.5 Activities that are not technically Human Subjects Research
Program Evaluation, Continuous Improvement, etc.
Many internal program evaluation, continuous improvement, and other activities occur in NYC public
schools that are not technically considered human subjects research. However, in some cases the NYC
DOE IRB may conduct a DOE Research Policy Review for non-HSR evaluations if they involve students
and are conducted by an external party who is not overseen by the DOE.
The non-HSR DOE Research Policy Review will consider alignment with DOE research policies.
Interviewing Central DOE Staff
If researchers are only requesting interviews with Central office staff about programmatic operations,
then full IRB review may not be required. However, we ask that the researcher provide the following
information to potential participants. These questions serve as a way for the interviewees to determine
if they want to participate, and disclosing this information aligns with ethical research practices.
• Name(s) of individual(s) from whom you would like to collect data (e.g., interview, survey)
• Topics that will be covered in the interview/survey (please attach a study instrument, if
available)
• Expected time commitment for the subject(s)
• A copy of an adult participant consent form (we can provide a template, if needed)
• A list of publications and/or other media outlets, if any, where you intend to distribute your
findings
• How the district and/or the research subject(s) will be represented in any published work
(identified by name, pseudonym, etc.)
Best Practices for internal non-HSR evaluation in the NYC DOE
For DOE staff conducting program evaluation, continuous improvement, or similar activities with
students in NYC schools, keep in mind these best practices:
• Ensure participation is voluntary
• Request and obtain informed consent from participants
• Consider the burden (effort, time, risk) on participants
• Prevent coercion by making sure to not ask people to recruit anyone they supervise
• Follow the DOE’s data security best practices [link coming soon]
• Do not compensate DOE employees for participation in evaluations
• Do not ask students sensitive questions

18
3.2.6 Research conducted by NYC DOE students -- Updated May 2024
Unfortunately, the NYC DOE IRB does not review research submissions from DOE students at this time.
Increasingly, NYC schools are offering courses in behavioral science research, providing students with
the opportunity to design and carry out research projects. The NYC DOE IRB affirms the importance of
students learning about the principles of behavioral science research and ethical treatment of human
research subjects.
The responsibility for reviewing students’ research projects, monitoring the development of research
protocols, overseeing data collection processes, and ensuring data security and disposal, should reside
with a school’s research course instructors and/or a faculty research review committees/school-based
IRB.
The DOE IRB has developed the basic guidelines to assist schools overseeing student research and data
collection. These guidelines embody many of the federal regulations governing research with human
subjects as well as policies and procedures that reflect the jurisdictional concerns of the NYC
DOE. Please see guidelines in the Appendix 6.3.
Again, the provided guidelines are recommendations, and the NYC DOE IRB does not review or approve
research conducted by NYC DOE students.
3.3 Types of Review
Depending on the type of study and the extent to which DOE affiliates are engaged in the research, the
DOE IRB may conduct different levels of review.
3.3.1 NYC DOE IRB Review
When the NYC DOE IRB is the IRB of Record
When the NYC DOE IRB is the IRB of Record, it will conduct a full human subjects research ethics review
of a study. This type of oversight usually applies to internal studies conducted by NYC DOE employees
doing research as part of their official work responsibilities. Sometimes the NYC DOE IRB may serve as
the IRB of Record in collaborative studies. Please reach out to the IRB inbox ([email protected]v) if you
are unsure if the NYC DOE IRB should be the IRB of Record.
Full Board Review
The NYC DOE IRB will likely conduct a full board review if the NYC DOE IRB is the IRB of Record for a
study.
Expedited Review
The NYC DOE IRB may conduct an expedited review if the study falls into the expedited categories as
detailed in §46.110.

19
Exempt Review
The NYC DOE IRB may determine a study is exempt according to federal regulations under §46.104, but
will still review for alignment with DOE research policies through an NYC DOE Research Policy Review.
3.3.2 NYC DOE Research Policy Review
When the NYC DOE IRB is not the IRB of Record
For submissions that have been approved by an external IRB, and where NYC DOE IRB is not the IRB of
Record, the NYC DOE IRB may conduct a DOE Research Policy Review. The DOE Research Policy Review is
a review for compliance with specific DOE research policies. We rely on the IRB of Record to thoroughly
review the study for compliance with human subjects protections, and require external
approval/exemption before we conduct a DOE Research Policy Review.
This type of oversight typically applies to external or collaborative research studies, where an external
IRB has already reviewed the study and is serving as the IRB of Record. This type of review is also used to
review non-HSR evaluations involving students, conducted by an external organization, and not initiated
on the DOE’s behalf.
DOE Research Policy Review
The NYC DOE IRB typically reviews all study submissions for compliance with DOE research policies,
irrespective of IRB determinations. The NYC DOE is a complicated environment for research due to the
presence of children (a vulnerable category of research subjects), and the unique context and hierarchy
of school buildings. As a result, the NYC DOE IRB requires compliance with additional policies in order to
protect individuals’ privacy and the work of the DOE. The NYC DOE Research Policy Review typically
applies to all submissions to ensure compliance with DOE policies, which are detailed in the DOE
Research Policies section.
DOE Research Policy Review can happen at different levels (which align with IRB “Full Board” and
“Expedited” review levels for logistical ease):
Board Meeting Review
Similar to Full Board Review, almost all new study submissions will be reviewed at a bi-monthly NYC DOE
IRB board meeting. Reviewers will read the submission, approve or identify required modifications in an
Issues Letter, and determine the next level of review (board meeting or accelerated review).
A submission is more likely to go to board meeting review if it includes any of the following populations
or procedures:
• Research involves students
• Research is greater than minimal risk
• Protocol requests a waiver of documented informed consent
• Protocol wants to video record anyone, or audio record children or classrooms
• Data collection procedures (interview, focus group, or survey protocols) ask sensitive questions
of children
• Protocol wants to collect any medical or biometric data
• Protocol was previously rejected by the NYC DOE IRB
20
• Submission is a “Protocol Violation, Deviation, Adverse Event, or Unanticipated Problem”
• Protocol includes any other elements that may elevate the level of risk to potential participants
in the DOE’s perspective
• An amendment significantly changes research procedures or level of risk
Accelerated DOE Research Policy Review
Similar to Expedited Review, the following types of submissions are likely to be reviewed as an
accelerated review:
• Minor amendments (adding research staff, uploading translated materials, adding schools)
• Resubmissions, amendments, or continuations if the board meeting review determined the
study qualifies for accelerated review

21
4. The NYC DOE IRB Process
4.1 Process Graphic
4.2 Review Process Steps
Each submission to the NYC DOE IRB follows the following review process steps:
1. Researcher submits protocol form through IRB Manager.
2. IRB Staff conducts pre-review/screening and either returns form to PI for revisions, assigns to
Full Board review (or DOE Research Policy Board Review), or assigns to Expedited review (or
DOE Research Policy Accelerated Review).
3. Study is reviewed by the Full Board or by Expedited Review, and reviewers submit
determination.

22
4. PI receives determination letter (Approval letter, Rejection letter, or Issues Letter with required
revisions).
5. If needed, PI makes modifications and resubmits.
4.3 Using IRB Manager
NYC DOE Data Requests and IRB submissions go through IRB Manager:
https://nycdoe.my.irbmanager.com/
4.3.1 Before you begin:
You must use your institutional email address to create an account. If you are a DOE employee but you
are submitting a research protocol for a graduate program, you must use your university email address
to create an account. You may not use a non-institutional email address to create an account (Gmail,
Yahoo, Hotmail, etc.).
If you already have an account but forgot the password, do not create a new account. Just reset your
password. If you haven’t logged in for a while, your account may be deactivated. If so, please email
not create a new account.
Specific requirements for IRB submissions:
• If you are a university student, your faculty advisor will need an IRB Manager account and
relevant CITI training in order to add them to your protocol.
• If you are a university student or external researcher, your institutional IRB will need an IRB
Manager account in order to be added to your protocol.
4.3.2 To create an account:
1. On the IRBManager login page, click on “Click here to register.”
2. Enter your institutional email address and confirm.
3. Fill in the relevant information about your organization/institution, name, and contact
information.
4. Click Register.
4.3.3 To submit an IRB protocol or Data Request:
After logging in, click on the “Start xForm” link under Actions in the top left corner. Select the type of
form you need to submit.
Available forms include:
• Credentialing – Use this form to submit CITI certification
• Data Requests – Use this form to submit a new Data Request
• IRB New Protocol Submission Form (v. 4.5) – Use this form to submit a new IRB protocol
Available forms for users with an open IRB protocol, accessed through your open protocol:
• Add or Remove Study Staff – Use this form to add or remove staff from your study

23
• Amendments Submission – Use this form to submit a minor modification to your study
• Closure – Use this form to close your study
• IRB Protocol Continuing Review/Renewal Request Application 2.0 – Use this form to renew
your study (studies are typically only approved for one year at a time)
• Protocol Violation, Deviation, Adverse Event, and/or Unanticipated Problem Report – Use this
form to report any protocol violations, deviations, adverse events, or unanticipated problems
If you need to make a major modification to your study, use the “Copy for Amend” function. Download
instructions from IRB Manager here: 4.5+ Amendment Instructions (this link requires being logged into
your IRB Manager account).
If you would like to see all of the questions on a form before you begin completing it, click on the Print
icon next to the form to access a view where you can print.
4.4 Submitting to IRB Manager
4.4.1 General Instructions
To start a form for a new study:
1. Log into IRB Manager.
2. Look in the top left corner of the window, for the menu under “Actions”
3. Click on the “Start xForm” link under Actions.
4. To start a new study protocol form, click on “IRB New Protocol Submission Form (v. 4.5).”
To access a form specific to an existing protocol (such as a continuation or amendment form):
1. Log into IRB Manager.
2. Go to your Dashboard.
3. Scroll to the bottom of the page and click on the blue number of the protocol where you want
to submit the new form.
4. Select “Start xForm” from the “Actions” menu on the left.
5. From here you can submit a minor amendment form, add/remove study staff, closure form,
continuation request, or a protocol violation.
Common issues with submitting a form
• If someone other than the study PI is submitting the form in IRB Manager, it will be sent to the
PI for final signature and submission. The submitter must click through the form all the way until
they click the “Submit” button to ensure the form has been fully submitted.
• Protocols submitted by students will be sent to the faculty advisor for final review and
submission.
• Protocols with the following status have not been fully submitted:
o Data Entry Stage
o PI Signature for Coordinator submission
o Faculty Advisor Approval

24
4.4.2 Protocol xForm
The xForm is the primary protocol form that researchers fill out to submit to the NYC DOE IRB. In this
form, submitters provide information about the proposed research study, including:
• Study information, documentation of institutional IRB approval, funding information
• PI and research study team personnel information
• Research locations
• Research questions
• Data collection methods, data collection tools
• Study subject populations, inclusion/exclusion criteria
• Risks/benefits to subjects
• Subject compensation procedures
• Recruitment procedures, screening procedures
• FERPA and PPRA requirements
• Consent and assent procedures
• Principal Permission
• Data confidentiality and subject privacy protections
Recommendations for successful submissions:
• Institutional Approval for External Studies
o All External studies must receive approval from the institutional IRB before submitting
to the NYC DOE IRB. The institution/university will serve as the IRB of Record for the
study.
• Brevity, clarity, and consistency
o Ensure answers to protocol questions are brief and clear. Text copied from grant
proposals or dissertation chapters is typically not appropriate to answer IRB protocol
questions. Please read the questions carefully and answer them completely. Incomplete
or unrelated responses will delay review.
o Ensure all elements of the protocol and attachments are consistent with one another.
Inconsistencies will delay review.
• Attachments
o Do not attach documents with unusual characters or symbols in the file name (such as
“|”)
o Do not upload blank attachments.
• Research Personnel
o List all study personnel on the protocol, along with up-to-date CITI certification. Please
note, the DOE IRB requires completion of the 26 DOE CITI courses. Please see CITI
certification for more information.
o All submissions must identify a contact person for the IRB of Record, usually someone
from the institutional IRB’s office. They will need to create an IRB Manager account.
o Student submissions must include the faculty advisor.
o DOE staff members submitting protocols for research being conducted outside of their
DOE job responsibilities (such as for an advanced degree) must adhere to the following
requirements:

25
▪ Use your university email address to create an account and submit the protocol.
Do not use your DOE email address.
▪ List the study as External. DOE employees or affiliates who are conducting
research to complete a graduate degree are considered external researchers.
Even though the researcher is a DOE employee, they are not conducting their
dissertation research on behalf of the DOE, so the study is considered external.
▪ Identify yourself as a student.
▪ Identify yourself as affiliated with the DOE.
▪ Include your Conflicts of Interest Board waiver letter.
4.4.3 Credentialing
Researchers can submit a Credentialing form to provide CITI certification. Please note, CITI certification
must adhere to NYC DOE IRB CITI requirements.
4.4.4 District Sponsor Request Form
[Coming soon.]
4.4.5 Data Request
[Coming soon.]
4.4.6 Amendment -- Updated May 2024
There are several kinds of amendments a researcher can submit, depending on the changes being
proposed. Please review the following to determine what kind of amendment you need to submit.
• Add/Remove Contacts Form
o To add or remove contacts from the research team, or change PI
o Must include CITI certificates
o Cannot change anything else about the form
• Administrative Amendment
o Simple amendment form – does not copy the entire protocol.
o This amendment can only be used for the following:
1. Uploading translated versions of documents that have already been approved
by the NYC DOE IRB.
2. Uploading signed principal letters (original principal letter must have already
been approved by the NYC DOE IRB).
3. Adding or removing schools from the study.
• Standard Amendment
o Copy for Amend – This will copy the entire protocol and you will need to update/revise
any sections related to your proposed amendment.
o This amendment must be used for any other proposed change to the protocol.

26
o Instructions to Copy for Amend (also available for download here:
https://nycdoe.my.irbmanager.com/Attachments/791883a7-a192-4f83-b52c-
7080a813896e)
1. Go to your Dashboard.
2. Scroll to the bottom of the page and click on the blue number of the protocol
where you want to submit the amendment.
3. Scroll to the bottom of the page and select the initial review event (“Initial
Submission”) and click on the blue text.
4. In the left-hand “Actions” column, select “xForms”
5. First the first “IRB New Protocol Submission Form” and click on the icon that is a
picture of a yellow folder with a green plus sign.
6. You should get a pop-up message saying, “Copy for Amendment?” Click OK.
7. Describe your amendment and attach appropriate documents. Click Next.
8. You will now see your original submission. Go through the original protocol and
update/revise appropriate sections in accordance with your amendment
submission.
• If you are changing documents, please attach versions with tracked-
changes as well as clean versions.
• Ensure all changes you are making to the protocol are explained in the
amendment description. Making additional changes to the protocol that
are not included in the amendment description will slow down pre-
screening and processing time, and your amendment may be returned
to you for clarification.
9. When you are finished with all revisions, click Submit.
Resubmissions or amendments that include changes to attachments must include versions of the
attachments that clearly show what has been changed. This can be shown either with tracked changes
or by highlighting. Please also include a clean version of the document.
4.4.7 Protocol Violation/Deviation
[Coming soon.]
4.4.8 Continuation -- Updated May 2024
Studies are approved for one calendar year at a time. If any study activities continue beyond one year
after the approval date, researchers need to submit a Continuation form to request another year of
approval. Continuation forms should be submitted 2-3 months prior to the study expiration date. If the
researcher needs to make any changes to the study, do not make them in the Continuation form. Submit
an Amendment form instead.
For complex multi-year studies in which procedures may change over time as the study evolves, we
prefer that researchers keep all related activities in a single submission and submit amendments and
continuations (rather than closing the study and submitting a new study with the updated procedures).
27
4.4.9 Closure
[Coming soon.]
4.4.10 Other processes
[Coming soon.]

28
5. NYC DOE Research Policies
The NYC DOE IRB may conduct a DOE Research Policy Review to ensure a protocol aligns with DOE-
specific policies, beyond the IRB review. The NYC DOE research policies apply to all studies within its
jurisdiction. While the Research Policy Review is not an IRB review, NYC DOE research policies are
heavily informed by federal research regulations.
5.1 Considering doing research in NYC public schools
5.1.1 Demonstrating value to the DOE -- Updated May 2024
Research must demonstrate alignment with the DOE’s priorities. See the Chancellor’s pillars here:
https://www.schools.nyc.gov/about-us/vision-and-mission/four-pillars-for-building-trust-in-nyc-public-
schools
Proposals to conduct any research with schools, students, or staff must demonstrate clear and direct
value to the NYC DOE. Researchers can demonstrate this in one of two ways:
1. Justify why the research needs to happen in NYC public schools by explaining how the research:
o Aligns with NYC DOE priorities, without duplicating existing work
o Will be used by the NYC DOE
o Provides direct benefits/services to students or schools
o Is different from other work that has been done on the subject
2. Provide evidence of support from a “District Sponsor” (a central office or superintendent office
staff member, or a principal in certain cases)
1. Justify why the research need to happen in NYC public schools
Justification that cites contribution to general knowledge or academic discourse as the value to NYC DOE
is not adequate. Participation in research requires using DOE resources, both tangible and intangible, so
proposed research must demonstrate value to the DOE that outweighs demands on DOE resources.
2. Provide evidence of support from a District Sponsor
In order to facilitate the use of research to inform policy and practice, we highly encourage researchers
to work with NYC DOE practitioners to ensure research is relevant, timely, and useful for decision-
making.
Researchers may demonstrate evidence of the value of their research to NYC schools through obtaining
a detailed letter of support from a District Sponsor from the central office, district, or school explaining
how the DOE will use the results to inform policies and decision-making.
• Strong letters of support demonstrate how the study aligns with the priorities of the NYC DOE,
what NYC DOE policies or programming decisions the study will inform, and how the NYC DOE
will access and use the study findings.
• Obtaining support from a District Sponsor does not guarantee study approval by the IRB.
• Securing a district sponsor does not commit the NYC DOE to using findings or indicate an
endorsement of study results.

29
We highly recommend that the following types of proposals secure support from a District Sponsor prior
to submission (and do not rely only on the justification explanation). Approval for these types of studies
with justification only, and without district sponsorship, is rare:
• Proposals from:
o Graduate students who are not DOE employees
o University professors
o Research firms
• Studies of interventions, curriculum, technology, or professional development
If a study requires access to FERPA-protected student data, a District Sponsor may be required in order
to meet the FERPA studies exception. See more information here: https://infohub.nyced.org/working-
with-the-doe/research-irb/faqs-for-external-data-requests
Exceptions
There are some limited cases where justification and/or district sponsorship may not be required for
NYC DOE IRB approval:
• Proposals from DOE staff members conducting dissertation research may not need support from
a District Sponsor, but must adhere to the additional requirements detailed later in this guide.
o Note, a District Sponsor may still be required to qualify for a FERPA exception for access
to student data.
• Certain required federal studies (NAEP, NTPS, ECLS, HS longitudinal study, school pulse panel,
SSOCS), may not require support from a District Sponsor. See here for more examples:
https://nces.ed.gov/surveys/
Reasons a study may not be approved
• Justification for why the study needs to happen in NYC Public Schools is inadequate, or cites
“contribution to general knowledge or academic discourse” as the primary reason.
• Study does not need to happen in schools or in the education context.
• The NYC DOE IRB reserves the right to reject submissions that have gone through multiple
rounds of feedback but remain incomplete or not in compliance with DOE policies or form
instructions.
Difference between a “Letter of Support” from a District Sponsor and the “Principal Permission Letter”
The Letter of Support from a District Sponsor can be collected prior to DOE IRB review and approval,
and is meant to be a demonstration of the value of the proposed research to the DOE. The letter of
support comes from a DOE staff person, and conveys how the research will help inform policy and make
decisions. The Letter of Support can also serve as evidence that the research is being done “on behalf of
the DOE,” which is necessary if the researcher intends to access FERPA-protected data and wants to use
the FERPA “studies” exception. The completed Letter of Support can be attached in the “Other
attachments” section of the IRB submission form.
The Letter of Support would typically come from a DOE central office, superintendent’s office, or
borough staff person. In rare cases where the research would only occur at a single school, a letter of
support may come from a single principal. The researcher may reach out to DOE
30
central/superintendent/borough staff with a proposed project to solicit a letter of support (while
making clear that the project has not been approved by the IRB yet).
Strong letters of support demonstrate how the study aligns with the priorities of the NYC DOE, what NYC
DOE policies or programming decisions the study will inform, and how the NYC DOE will access and use
the study findings.
The Principal Permission Letter is collected after DOE IRB review and approval. An unsigned draft of this
letter should be attached to the DOE IRB submission form for review and approval.
The principal permission letter must include detailed information about the study, including:
• Research questions, design, and methodology
• Recruitment process
• Participant burden
• Confidentiality/anonymity
• Risks/benefits
• Uses of the data
• Anything needed from the school (such as, identifying a space to conduct interviews, a location
to post a recruitment flyer, approval to conduct an intervention tied to the research)
• Signature line and date for the principal to give their permission to conduct the study in their
school, including the title of the study, protocol number, and the school name
While the NYC DOE IRB may approve a study, it is still ultimately up to the principals to give permission
for the research to happen in their schools. Approval by the NYC DOE IRB does not guarantee access to
any particular school, individual, or data. The researcher is responsible for making appropriate contacts
and getting the required permissions and consents before initiating the study.
Researchers may discuss their study with principals before official NYC DOE IRB approval, but they may
not collect the formal principal permission letter until the study has been approved by the NYC DOE IRB.
After the researcher has received NYC DOE IRB approval, they may contact principals to request formal
permission to conduct the study in their schools.
Overall, we require external submissions to demonstrate the overall value of their study to the NYC DOE
prior to review by the NYC DOE IRB. One way to do this is through a Letter of Support. Then, once a
study has been approved, researchers would approach individual school principals to obtain permission
to conduct their specific, IRB-approved study procedures in that school.
5.1.2 Burden
[Coming soon.]
5.1.3 Equity
[Coming soon.]

31
5.1.4 In-person or Virtual Studies -- Updated May 2024
While strict COVID restrictions on in-person research are no longer in effect, the use of virtual or online
study procedures remains common and the NYC DOE IRB does allow for both virtual and in-person study
activities. As a result, NYC DOE IRB asks that researchers clearly explain what activities would occur in-
person and what would occur virtually, and ensure this is clearly communicated in the protocol
submission and all materials given to participants.
The NYC DOE IRB also requires study personnel to seek out and follow all school visitor policies and
procedures (including health and safety protocols) when entering school buildings.
5.1.5 Study Personnel -- Updated May 2024
Principal Investigator
[Coming soon.]
Research Study Staff
[More coming soon.]
Tele-recruiters
Researchers must include tele-recruiters in the Research Staff table in the submission form. Some of the
larger federal survey studies may decide to identify dedicated NYC-specific recruiters to ensure they
follow our recruitment policies. Since these tele-recruiters will not be entering schools or interacting
with students or data, they likely will not need to go through the PETS security clearance process. Please
see the section on Security Clearance below.
Graduate student research
The NYC DOE IRB considers graduation student research submissions on a case-by-case basis.
Graduate student submissions:
• Must submit a detailed, thorough, and complete IRB application. If a submission does not
answer all questions, or clearly has not complied with directions in the form, it will be rejected.
• Must include evidence of review from the university/college IRB.
o Please note, even if your institution’s IRB has determined your project to be exempt,
expedited, or not human subjects research, you are still required to submit to the NYC
DOE IRB for review of compliance with NYC DOE research policies.
• Must demonstrate strong support from a faculty advisor, including support completing and
submitting the NYC DOE IRB application.
• Must not put undue burden on NYC public schools, students, families, or staff.
Some graduate programs require extremely fast timelines for thesis or dissertation completion. The DOE
IRB’s timelines for reviewing and approving studies often do not align with the requirements of these
programs. The DOE IRB will not expedite protocol review due to fast graduate program timelines. If your
graduate program requires a very fast thesis/dissertation timeline, we recommend finding research
opportunities elsewhere.
32
Submissions from graduate students who are also DOE employees must follow additional policies:
• DOE employees must obtain a determination from the NYC DOE Ethics Office or NYC Conflicts of
Interest Board and attach the letter to the IRB application.
• DOE employees conducting research for an advanced degree cannot use student data or other
data collected through their employment for research without a COI waiver, an approved Data
Request, and a signed data non-disclosure agreement (NDA) executed with their
university/institution.
• DOE employees conducting research for a degree may not engage in research activities or
collect data for research without approval from the NYC DOE IRB.
• In rare cases, a DOE employee may request to use data previously collected as part of their
normal work activities for a research project. These requests are reviewed on a case-by-case
basis.
The timeline of review of dissertation studies depends on a few things:
• Is the student a current DOE employee?
o If yes, they do not have to demonstrate value to the DOE, since a DOE employee gaining
advanced education is a value in itself. They will still need to explain the value of their
study in the application, but they are not required to provide a letter of support from a
district sponsor.
o If no, they should plan to obtain a letter of support from a district sponsor in the DOE to
demonstrate the value of their research. If they do not submit this letter in their initial
submission, the application will be returned to them, adding time to the process.
• Does the study involve direct data collection from students (surveys, interviews, focus groups,
observations, etc.)?
o If yes, studies involving direct data collection from students are typically reviewed by
the full board.
o If no, the study may be reviewed expedited if it only involves data collection from
adults. (However, if an adult-only study potentially poses increased risk to participants,
as determined by the DOE IRB, it may still go to the full board.)
• Does the study involve using individual-level student administrative data (either requested from
the DOE, or in a person’s possession through their work with students as a DOE employee)?
o If yes, studies involving individual-level student data need to go through some additional
steps (adding time to the process):
▪ The student must submit and received approval of a Data Request
▪ The student may need to obtain a DOE District Sponsor to meet the FERPA
exception requirement
▪ The student's institution would need to sign the data non-disclosure agreement
(NDA)
Conflicts of Interest Board
If you are affiliated with the NYC DOE, but conducting research outside of your DOE job responsibilities,
such as for an advanced degree, you must contact the NYC DOE Ethics Officer, Ms. Samantha Biletsky
(SBiletsky@schools.nyc.gov), prior to submitting to the NYC DOE IRB to determine if a Conflict of
Interest Exemption is required for the proposed research.

33
See here for more information about Conflicts of Interest, and to access the Research Waiver Request
Form. (DOE login credentials are required to access this page.)
Note that the DOE rarely permits DOE staff to conduct research in their own school or with students,
parents, teachers, or other staff that are under the supervision of or in a position of subordination to
any member of the study team.
CITI Training
CITI Training Requirements
The NYC DOE IRB requires all researchers to complete the 26-module Social & Behavioral Research –
Basic/Refresher course (ID 184110) that is affiliated with the NYC DOE. Researchers must affiliate
themselves with the NYC DOE in CITI in order to access and complete this course. Documentation of
completion of the NYC DOE affiliated course must be provided for IRB review at the time of protocol
submission, or prior to submission using the Credentialing xForm in IRB Manager. Certificates of
completion of the NYC DOE course are valid for 5 years, after which point a Refresher course will be
required.
If you have used the same email address to create your IRB Manager account and to complete the CITI
training with the NYC DOE, your course completion records will automatically upload into your IRB
Manager user profile, and will show up in any form you submit. This is the preferred method. However,
if you used a different email address or your records are not transferring over for some reason, please
submit a Credentialing form to upload your completion certificate manually.
If your research is subject to FERPA, select the Family Educational Rights and Privacy Act (FERPA) course
for question 4. If no part of your research is subject to FERPA, select the Information Privacy Security
(IPS) course most applicable to you.
All parties are strongly encouraged to complete the Conflicts of Interest course, although it is not
required. If you are a DOE employee, or otherwise affiliated with the DOE, the Conflicts of Interest
course is recommended, and may be required.
If your institution has a CITI or other training program that you would like to use as a substitute, please
submit a request to [email protected]v that includes the list of courses and demonstrates how these
courses align with the DOE requirements. To note, the course list must align with the DOE course
requirements, including the 26 modules (see list of modules in the Appendix).
Faculty advisors only need to have the full NYC DOE CITI training if they will be engaged in any research
activities for this proposed study, or working with any study data from this study. Engaged in research
activities could include recruiting participants, consenting participants, conducting data collection, or
reviewing data collected from research participants. If faculty advisors will be engaged in research
activities, they will also need to be listed on the protocol under “Research Staff.” If faculty advisors will
not be engaged in any research activities for this study, CITI certification from their home institution is
adequate.

34
Accessing CITI Trainings
Researchers who are submitting protocols to conduct research in NYC schools may complete CITI
trainings as a NYC DOE affiliate. In your CITI account, researchers may affiliate themselves with the NYC
DOE in order to access our required courses. Please see instructions below.
1. Access the CITI website here: https://about.citiprogram.org/en/homepage/
2. Log in or Register
a. If you have an existing CITI account, log in and follow instruction to add the NYC DOE as
an affiliated institution.
i. In the blue “Welcome” banner, click “Add Institutional Affiliation”. Enter “New
York City Department of Education” and follow instructions. You may affirm you
are an affiliate of the NYC Department of Education, since you are applying to
conduct research with us.
b. If you do not have an existing account, click “Register” and set up an account with “New
York City Department of Education”. You may affirm you are an affiliate of the NYC
Department of Education, since you are applying to conduct research with us.
3. Navigate to the Homepage and click on “View Courses” for New York City Department of
Education. Follow instructions on CITI training instructions document.
Security Clearance and PETS
PETS, Security Clearance, and Fingerprinting
All personnel named in an approved IRB protocol who are designated to conduct research in NYC public
schools, with NYC DOE staff or students, or using NYC public school student data, must complete the
NYC DOE security clearance process.
Research staff are required to have NYC DOE security clearance if they are doing any of the following:
• Entering schools
• Interacting with students in person
• Interacting with students virtually
• Accessing student direct identifiers*
• Accessing teacher direct identifiers* (if data includes evaluation data)
*Direct identifiers may include name, date of birth, address, contact information, and non-coded ID
number
The NYC DOE security clearance process involves the following steps:
1. PI receives IRB approval from the NYC DOE IRB, including approval letter naming all research
team member names.
2. For each research team member, email required information and scanned copies of required
documents to IRB@schools.nyc.gov, including:
a. Stamped DOE IRB Approval Letter
b. Government Issued State ID
c. Signed Social Security Card
d. Current email address and phone number

35
3. The DOE IRB will verify this information and enter it into the PETS system.
a. Note: We understand individuals may feel uncomfortable emailing SSN information. If
so, please state in your email that you would like to verify your SSN over the phone and
we will set up a call.
4. Within 7 business days, researchers will receive a nomination email from
PETSAdminSup[email protected]. This email will outline all next steps in the security
clearance process, including login access to Applicant Gateway where all required forms can be
found.
5. Researchers must complete the required steps and forms in the Applicant Gateway. This may
consist of completing fingerprinting and/or a Background Questionnaire (see below for more
information about fingerprinting).
The PETS system does not send any confirmation to researchers if they become eligible. It is the study
PI’s responsibility to ensure all research staff contacting schools or entering schools are fully eligible in
PETS. Please contact [email protected]v if you have questions.
The NYC DOE takes the safety of our students, staff, and schools very seriously. The security clearance
process is not optional. If researchers do not follow the NYC DOE’s requirements for security
clearance, the study may be paused or terminated, the IRB of Record will be notified, funders may be
notified, future studies may not be approved, and the Office of Special Investigations may be
involved. It is the PI’s responsibility to ensure all research staff are cleared to enter schools.
If a researcher’s eligibility status changes, and they become ineligible for some reason, the NYC DOE IRB
will contact the study PI to inform them and to direct them to remove that researcher from schools
immediately.
Fingerprinting
A researcher may need to complete fingerprinting for the security clearance process. If so, once all
required forms are completed and signed, follow the directions provided in the Applicant Gateway to
schedule the fingerprinting appointment through IdentoGo. Please note:
• Fingerprinting is no longer conducted at 65 Court Street.
• Fingerprinting is conducted on an appointment-only basis.
• The cost for fingerprinting is currently $101.75.
What if I’m already eligible in PETS?
Security clearance is role-specific, so individuals are cleared for specific roles and vendors. Any
researchers doing research in DOE schools must be entered into PETS on the IRB vendor roster. If
researchers already have fingerprints on file with the NYC DOE IRB, or are already eligible in PETS under
a different vendor’s roster, they still need to submit the required documents to the IRB inbox and
complete any required steps. The researcher may not need to be re-fingerprinted, and they may just
need to update responses in the Background Questionnaire. If a researcher is already in PETS but not
on the IRB vendor list, they are not cleared to enter schools for research. They must be found eligible
on the IRB vendor list.

36
• For example, an individual may be eligible in PETS under a vendor who provided tutoring
services in schools a couple years ago. In this case, the individual may be “Eligible” under the
vendor “ABC Tutoring, LLC”. This does not mean this individual is cleared to enter schools for a
research project. They must be listed as “Eligible” under the vendor “Institutional Review Board”
to be able to enter schools for research.
What if a researcher does not have an SSN?
Researchers must have an SSN in order to be entered into PETS and to obtain security clearance. We
understand that some research studies include international university students who do not have SSNs.
Unfortunately, there is no option at this time to add these individuals into PETS under the IRB vendor.
5.1.6 Research Design -- Updated May 2024
Quality of research
[Coming soon.]
Randomized control trials, control groups
Studies that involve student-level randomization within a school are considered on a case-by-case basis
and are rarely approved by the NYC DOE IRB. In a limited number of cases, the NYC DOE IRB may
approve a study using randomization between schools.
The NYC DOE IRB’s policy on the use of randomized controlled trials considers both methodological
challenges and ethical concerns.
Methodological Considerations
• Educational systems like the New York City public schools are highly complex organizations, and
it is challenging to adhere to the strict inclusion/exclusion criteria and intervention
implementation requirements in this environment. Specifically, the following methodological
challenges often occur in public school systems:
o Variables can rarely be controlled tightly.
o Contamination of effects can compromise randomization, especially where student
interaction is moderate to extensive.
o Blinding, a cornerstone of clinical RCTs, is virtually impossible in studies conducted in
schools.
o Schools are particularly unique settings, and a randomization design may not actually
control for the contextual factors that may impact outcomes.
o Interpreting results of RCTs in schools is not as simple as saying, “It works,” or, “It
doesn’t work.” (e.g., if it works with low-income students in the rural south, does that
mean it will work with middle-income students in the urban north?)
Ethical Considerations
• In the public school setting, RCTs that deny services to a control group that has been created
solely for the purpose of the research experiment are ethically and administratively
problematic. Distribution of services or benefits that appears to be arbitrary, or random, may be

37
perceived as unfair in the school environment, where instruction and interventions are often
differentiated based on carefully considered student needs, not randomly.
How the NYC DOE IRB considers RCT designs:
Rationale for the design
• The Board will consider:
o Is there is a clear scientific and policy rationale for using an RCT?
o Would another research methodology produce the desired outcome?
o Does the research design address an important policy or practice question that is a
priority for the DOE and participating schools (e.g., will it lead to increased school
attendance, improved educational outcomes)?
• Recommendations:
o Provide a clear and detailed explanation of the proposed design in your submission.
o Consider other study designs (see below).
Strength of the study versus risk/burden on participants
• The Board will consider:
o Is the margin of improvement large enough to warrant an RCT?
o Does the proposal communicate clear thinking about indicators (e.g. How will
improvement be measured? How will data on the indicators be collected?)?
o Does the proposal communicate a deep understanding of the sample: Who is the target
population? Is the sample large enough to make it really random? Is the sample
representative of the larger population? When will random assignment take place
within schools? Following randomization, are the treatment and control groups
comparable along important indicators?
o Does the potential benefit to students/schools outweigh the concomitant burden to
school staff of the sample size/time commitment required for an RCT?
o Is the proposed study population particularly vulnerable? Would the RCT result in
inequitable educational opportunities for students within the same school?
• Recommendations:
o Make a strong case for why the study must be designed in this way.
o Clearly explain the intended participant population, including why it was selected and
how you know it will yield a strong design.
o Justify the exclusion of some students from receiving the intervention (some ideas: RCT
design is required by study funder and intervention wouldn’t be possible without the
study funding, low data collection burden on students, many students will still receive
the intervention through the delayed treatment model, etc.)
o Consider ways to provide equivalent experiences for control populations.
Feasibility of the study design
• The Board will consider:
o Would the school principal agree to an RCT in their school? School leaders need to be
able to make instructional decisions in the best interest of their students, and an RCT
38
design might prevent school leaders from providing students with the services they
need.
o Is the RCT implementation logistically feasible given the complicated scheduling in
schools?
o Will adequate resources be available for the development/implementation phase of the
program, including:
▪ Attention and sensitivity to stakeholder perspectives/concerns: recruitment
activities that consider the position of the control group; honest discussion of
what will be expected and potential risks and benefits; adequate time for
control schools to decide whether it is worth it to participate.
▪ Careful piloting of recruitment/informed consent procedures
▪ Respect for the need for tight control of the randomization process
• Recommendations:
o In your submission, explain the logistical feasibility of the research design. Demonstrate
an understanding of the school’s operations and how the research design would fit in.
o Provide evidence that the school leader is likely to agree to participate in the research
design. Please note, this does not mean that the principal just agrees to participate in
the intervention, but that they also understand the design and agree to random
assignment.
o Explain how you will implement attentive and sensitive recruitment and implementation
procedures that allow for informed consent and adherence to a randomization process.
Consider other study designs:
• Offer the intervention to the control group at the conclusion of the study.
o If timing prevents offering the intervention to students in the control group (due to
student matriculation), offer it to control schools the following year.
• Offer something equivalent to the control group (such as, access to resources, instruction,
compensation, etc.).
• Delayed treatment
o Offer the intervention or treatment condition to the control group on a delay, either
later in the year, or the following year.
• Phase-in treatment
o For example, the study is run twice within the school year, once per semester. Students
or schools who did not receive the intervention in the first semester would begin
receiving the intervention in the second semester.
• Rotation design
o For example, assuming a study with two groups, one group is assigned treatment and
one is control. Then those roles switch, with the previously treated becoming the
control and the previously controlled becoming treated.
• Multiple treatments
o Offer multiple treatments and test them against each other.
• Take advantage of existing randomization that occurs as part of the operations of the school
system
39
o For example, random selection is used in the NYC DOE student admissions process. As
part of the admissions process for most DOE public school programs, each applicant is
assigned a random number, as in a lottery. These random numbers are used in cases
where there are more applicants than seats available at a specific program.
• Alternating treatment (combine rotating and delayed treatment)
o Between two groups of schools, assign alternating grades to treatment/control each
year, and include follow up treatment to control students in the following year.
Interruptions to instructional time
In almost all cases, research activities cannot take place during or in any way interfere with instructional
or professional development time. If approved, researchers should work with school staff to find an
appropriate time and location to conduct research activities.
In very rare cases, researchers may justify a proposed interruption of instructional time or required
classroom activities. However, this is rarely permitted.
Research on programs, interventions, professional development, or other programs
When a research project is connected to an intervention or program, the IRB submission must carefully
explain the following:
• The intervention activities
• The research activities
• If participation in research is required for participation in the intervention.
• What data would be collected for the intervention only, what data would be collected for
research only, and what data would be collected for both the intervention and for research.
• Participant recruitment and consent materials must make clear what activities are part of the
intervention, what activities are part of research, and if research is required for participation in
the intervention.
o If the research is not required for the intervention, the researcher may want to use two
separate consent forms, one for agreeing to participate in the intervention, and another
form for agreeing to the research.
In cases where participation in research is required for participation in an intervention, the Board will
carefully consider the risks and benefits of the project to determine if the intervention could be offered
without the research, and to ensure students/families/staff are not being denied an opportunity if they
do not agree to participate in the research.
Product testing
The NYC DOE IRB does not typically approve research that is considered product testing. Research may
be considered product testing if any of the following apply:
• School/site inclusion criteria are based on convenience or cost considerations for the researcher
• Subject inclusion criteria are characteristics of the market or audience where the researcher
plans to market a product resulting from the research

40
The NYC DOE IRB may approve research that could be considered product testing if there is adequate
justification for the benefits to NYC public schools, the research directly benefits NYC public schools or
participants, or there is demonstrated support from the NYC DOE.
Risks and Benefits
The NYC DOE IRB takes a fairly conservative stance on risks and benefits, given that study subjects within
our jurisdiction include children and their families, and these children are legally obligated to be in
school. As such, the NYC DOE IRB requires researchers to thoroughly explain and justify the anticipated
risks and benefits of research. The Board will consider if the risks are justified.
The NYC DOE IRB is particularly concerned about certain types of risk, including risks of:
• Psychological harm (discomfort, embarrassment, worry, anxiety)
• Physical harm
• Legal harm
• Financial harm (damage to employability, insurability)
• Social harm (damage to reputation)
• Breach of confidentiality
• Breach of subject privacy or anonymity
Note: Collection of sensitive data including subjects’ religious affiliation, sexual history, drinking/drug
behaviors, discipline history, mental health status, or other private information may expose participants
to the above risks.
For all the risks identified, researchers must explain:
• The probability of risk (how likely is it that harm will occur)
• The magnitude of harm (how severe would harm be if it happened)
Minimal risk means the probability and magnitude of harm or discomfort anticipated in the research are
not greater in and of themselves than those ordinarily encountered in daily life or during the
performance of routine physical or psychological examinations or tests.
Studies involving greater than minimal risk
[Coming soon.]
Benefits
[Coming soon.]
5.1.7 FERPA & PPRA
Please review the Data Request FAQs here for more information: https://infohub.nyced.org/working-
with-the-doe/research-irb/faqs-for-external-data-requests
[More coming soon.]

41
5.1.8 Data Security
[Coming soon.]
5.2 Recruitment and Consent
5.2.1 Research site inclusion/exclusion criteria
Please explain in detail how you are selecting the sites for the research. School inclusion criteria such as
having “a diverse student population” is not adequate. Convenience sampling is rarely approved.
5.2.2 Principal Letter -- Updated May 2024
Researchers are required to get permission from the principal in order to conduct research in a school. If
research is about a school but the research activities would happen away from the school, researchers
may still be required to get principal permission to do the research.
While the NYC DOE IRB may approve your study, it is still ultimately up to the principals to give
permission for the research to happen in their schools. Approval by the NYC DOE IRB does not guarantee
access to any particular school, individual, or data. The research PI is responsible for making appropriate
contacts and getting the required permissions and consents before initiating the study.
The principal permission letter must include detailed information about the research, including:
- Research questions, design, and methodology
- Recruitment process
- Participant burden
- Confidentiality/anonymity
- Risks/benefits
- Uses of the data
- Anything needed from the school (such as, identifying a space to conduct interviews, a location
to post a recruitment flyer, approval to conduct an intervention tied to the research)
- Signature line and date for the principal to give their permission to conduct research in their
school, including the title of the study, protocol number, and the school name
You may discuss your research with principals before official NYC DOE IRB approval, but you may not
collect the formal approval letter until your research has been approved by the NYC DOE IRB.
After you have received NYC DOE IRB approval, you may contact principals to request formal permission
to conduct research in their schools. When contacting a principal to request permission to conduct
research, please send the approved stamped principal permission letter along with the NYC DOE IRB
stamped approval letter. Each principal agreeing to participate must sign the principal permission letter.
After obtaining signed principal permissions letters, please submit an amendment to your protocol in
IRB Manager attaching the signed letters.

42
The principal permission letter can be addressed to and signed by a site director if the site is a pre-K
NYCEEC or CBO-run center.
Please note, Principal Permission is different from principal consent to participate as a research subject.
Principal permission is to get permission from the principal to conduct research in their school and with
their students/staff/families. Principal consent is to participate as a research subject and participate in
data collection activities for the research.
5.2.3 Participant Recruitment -- Updated May 2024
Recruitment of research participants
The NYC DOE IRB has specific rules and recommendations for recruiting in schools.
In the submission form, provide a detailed description of the proposed recruitment activities. To prevent
delays in review time, please explain exactly who will be asking whom for what, where, and when?
• Who: Who is contacting potential subjects? In what context?
• Whom: Who is being contacted as a potential subject? Are you contacting teachers first,
then students?
• What: What is being asked of potential subjects? What are the steps? Take home a flyer?
Click on a link? Sign a consent form? Give verbal consent? Take a consent form home?
Provide information for screening?
• Where: Where are potential participants being contacted? How are they being accessed (in
person, email, etc.)?
• When: When is this happening? How long do potential subjects have to consent? Do they
take a flyer home and return it later? Do they consent in the moment?
The following recruitment activities are typically not allowed:
• Recruitment materials may not come from a person is a position of authority over the potential
research subject. NYC DOE staff may not send research recruitment materials to subordinates or
anyone over whom they have authority.
o For example, researchers may not ask principals to assist them in identifying and
recruiting school staff to participate in their study, since the principal has authority over
school staff, and a principal recruiting for a research study may be coercive.
• Researchers may not recruit research subjects using contact information that was obtained or
accessed for another purpose. NYC DOE employees or other affiliates who have proprietary
access to internal email lists may not use this access for the purposes of study subject
recruitment. Please note, teacher emails are rarely posted publicly, and the DOE does not
typically share staff contact information externally.
o If a DOE employee is conducting research outside of their DOE work (such as completing
a dissertation), they may not use their DOE position to access email addresses.
• If another organization has collected DOE subject contact information for their own purposes
separate from the research, they may not share that contact information with research staff for

43
recruitment. Instead, the other organization may passively forward a recruitment email to the
DOE staff members, who could then reach out to the research team if they are interested.
o For example, an organization has provided professional development to teachers and
has a list of teacher email addresses collected when teachers signed up for the PD.
Research was not mentioned when the teachers signed up for the PD. Later on, a
research firm is interested in studying the impacts of the PD program. The PD
organization may not share the teacher emails with the researchers for recruitment.
Instead, the PD organization may passively forward a recruitment email from the
researchers.
• Researchers may not ask participants for other people’s contact information, even in the case of
snowball sampling.
• Researchers cannot offer rewards to children for returning parent consent forms.
The following recruitment activities are typically allowed after approval by the NYC DOE IRB:
• With permission from the school principal, researchers may post a flyer in the school staff
lounge, distribute flyers in staff mailboxes, request a meeting with school staff, or ask a staff
member who is not in a position of authority to passively forward recruitment materials to
school staff.
o For example, a school office staff member may forward a recruitment email from a
researcher to the teachers.
• With permission from the school principal and classroom teacher, researchers may provide
recruitment materials to be sent home to families in students’ backpacks.
• Provide materials with easy ways to contact the researcher to opt in to a study, such as
researcher email, or flyers with a QR code that links to an interest form.
• Researchers can ask participants to passively forward information about a study to others, as
long as they do not supervise or hold any authority over those people (to avoid coercion).
• After obtaining NYC DOE IRB approval for the study, researchers can recruit NYC DOE school
staff using publicly available email addresses.
What does it mean to “passively forward”?
The NYC DOE IRB often recommends having someone passively forward recruitment materials, but what
does this mean?
• Forward means to send an email along to someone else without including the original sender
on the message.
• Passively means to not add any personal message or encouragement to participate.
o Messages like “FYI – research study” or a blank message would be considered passive.
o Messages like “Please sign up to participate in this study! It will be an amazing
opportunity for our school!” or “Review and complete the attached consent form.”
would be considered not passive and would not be allowed.
How does it work?
• A school staff member may receive an email from the researcher with information or
attachments about the approved study. The staff person can then forward the email directly to
other people, with a blank or passive message in the email body. Interested recipients can then
reach out directly to the researcher to express interest in participating in the study.

44
Notes about other recruitment methods
• Snowball sampling
o Researchers may not ask participants for other people’s contact information.
o Researchers may ask participants to passively forward information about a study to
others, as long as they do not supervise or hold any authority over those people (to
avoid coercion).
• Entering schools
o Researchers must be cleared by the NYC DOE IRB and the Office of Personnel
Investigations before they can enter schools. This includes completing fingerprinting and
clearing the background check.
o Researchers must obtain approval from the school principal before entering the school
to conduct recruitment or research activities.
o Researchers must follow all school visitor policies, including COVID precautions.
o Recruitment activities in schools must not interfere with regular school activities.
o Any recruitment activities happening in schools must be detailed in the IRB protocol and
principal permission letter.
• Accessing student roster data
o In some cases, researchers may request student roster data in order to facilitate
research recruitment. Certain studies may qualify to request roster data for screening or
recruitment. However, roster data is not typically shared with external researchers. It
may be shared in rare cases (such as, for federal studies with access to student data
under the FERPA audit exception, or other circumstances). In order to access student
roster data, the researcher must submit a data request to the NYC DOE, must qualify for
a FERPA exception, and must sign an NDA.
• Accessing NYC DOE staff contact information
o The NYC DOE and its staff cannot share staff contact information with researchers.
• Accessing publicly available contact information
o Researchers may use publicly available contact information (such as email addresses
posted on school websites) to recruit potential participants, as long as this is approved
by the NYC DOE IRB and the school principal, as applicable. Researchers may use public
principal emails to request permission to conduct research at their school, after NYC
DOE IRB approval.
• Using social media to recruit
o Researchers may use social media to recruit research participants. However, this
recruitment must align with the following guidelines:
▪ Researchers who are not doing research on behalf of the NYC DOE may not use
DOE-managed social media accounts to recruit for research.
▪ Research that is about NYC public schools, students, families, or staff must be
reviewed and approved by the NYC DOE IRB, even if recruitment is not
happening in schools, and would happen entirely through social media.
▪ In social media recruitment posts in groups or on personal social media,
researchers must identify themselves as researchers.
▪ If posting in a private group, the researcher may need to obtain permission from
the group moderator before posting.

45
• Advertising compensation in recruitment materials
o Compensation cannot be used as a recruitment enticement. If research participants will
be compensated for their participation, this may be stated on recruitment materials, but
it cannot be in the title or heading of any recruitment materials. Statements in
recruitment materials about the compensation must accurately describe the ways
participants can receive compensation.
▪ Bad example: “Sign up for this research study and receive $25!”
▪ Good example: “Sign up for this research study to share your perspectives!
Eligible participants who complete the survey will receive $25 for their time.”
• Accessing school staff contact information from professional development sessions
o [Coming soon.]
Researchers may not retain students’ contact information for other future research projects.
Researchers may retain student contact information for future contact within the same study, if this is
approved in the parental consent form.
5.2.4 Participant Screening -- Updated May 2024
Screening is when you obtain information from a potential participant in order to determine if they are
eligible to participate in your study. Screening activities start the moment the investigator obtains
information about the prospective participant to determine if they are eligible for the research.
Screening individuals to obtain and record information to determine eligibility involves obtaining
identifiable private information and is considered human subjects research for research subject to 45
CFR 46.
Screening may require getting informed consent, since you are obtaining identifiable private
information. You may be eligible to request a waiver of consent for screening procedures.
The NYC DOE IRB may approve a research proposal in which an investigator will obtain information for
the purpose of screening, recruiting, or determining the eligibility of prospective subjects without their
informed consent if either of the following conditions are met:
• The investigator will obtain information through oral or written communication with the
prospective subject, or
• The investigator will obtain identifiable private information by accessing records.
Certain studies may qualify to request roster data for screening or recruitment. However, roster data is
not typically shared with external researchers. It may be shared in rare cases (such as, for federal studies
with access to student data under the FERPA audit exception, or other circumstances). If you plan to use
roster data, you must list it in the “Study Description” section of the submission form in IRB Manager,
under the question that asks about “the use or access of existing data.”
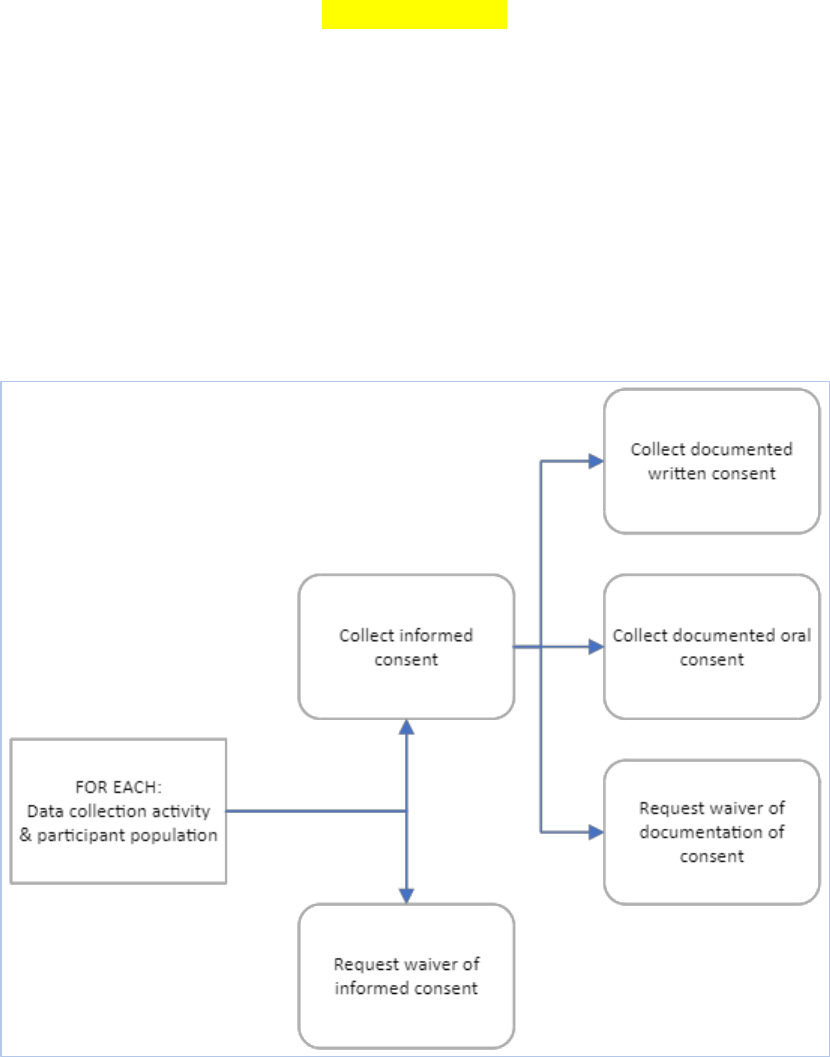
46
5.2.5 Participant Consent -- Updated May 2024
General consent policies
In your study proposal, you must explain your consent procedures for each data collection activity and
each participant population. For each activity and population, you will need to determine the
appropriate consent procedures. The NYC DOE IRB takes a conservative stance on participant consent.
Even if a study is not officially human subjects research, we expect submissions to account for consent,
either by collecting consent or providing a strong justification for requesting a waiver of consent.
You may collect informed consent or request a waiver of informed consent. If you are collecting
informed consent, you may collect documented written consent, documented oral consent, or you may
request a waiver of documentation of consent.
Consent Decision Chart:
The NYC DOE IRB requires researchers to account for specific consent and assent procedures for each
research subject population. This may include collecting consent/assent or requesting a waiver. See the
chart below.

47
Research submissions must provide the following consent/assent procedures for the following
participant populations, or request waivers for each:
Required Consent/Assent Procedures or Waiver
Study Participants
Adult consent
Parental consent
Child assent
Adults
X
DOE students 18 or older
X
X
DOE students under 18
X
X
Consent Form Templates
The NYC DOE IRB provides adult and parental consent form templates with all necessary sections for
researchers’ use. We recommend using the DOE consent form template to ensure you include all
required sections. Please modify the template as needed to reflect your study. Do not upload signed
consent forms into IRB Manager.
Ensure the consent form includes contact information for both the home institution IRB and the NYC
DOE IRB (IRB@schools.nyc.gov).
If you plan to audio or video record, the consent form must have a separate consent signature line
specifically for recording.
If you intend to retain deidentified data for future use, include this in the consent form.
Informed Consent
Before involving a human subject in research, the researcher must obtain informed consent from the
subject. The basic elements of informed consent are described in §46.116 and summarized here.
Prospective research subjects must be given the opportunity to discuss and consider whether or not to
participate in the research. The consent process should minimize the possibility of coercion or undue
influence. Adult and parental consent forms must be written in plain language that is understandable to
the subject, and must not exceed the 8
th
grade reading level.
There are several ways a researcher can collect consent:
• Documented written consent – provide prospective participants with a comprehensive consent
form, and the participant signs on paper or electronically
• Documented oral consent – provide prospective participants with a short form stating that
elements of informed consent were presented orally, participant signs on paper or
electronically, researcher must provide written summary of what is to be said to the participant
• Waiver of requirement to collect documented signed informed consent form – to qualify for a
waiver, the researcher must meet IRB requirements, and must still provide a written document
to participants
These methods are described in further detail below.

48
An IRB may waive the requirement to obtain informed consent for research if the IRB finds and
documents that:
• The research involves no more than minimal risk to the subjects;
• The research could not practicably be carried out without the requested waiver or alteration;
• If the research involves using identifiable private information or identifiable biospecimens, the
research could not practicably be carried out without using such information or biospecimens in
an identifiable format;
• The waiver or alteration will not adversely affect the rights and welfare of the subjects; and
• Whenever appropriate, the subjects or legally authorized representatives will be provided with
additional pertinent information after participation.
Note, a waiver of parental consent for access to student administrative data is never granted. (If a FERPA
exception is applicable, parental consent is not required, so would not require a waiver.)
Documented Written Consent
Most commonly, informed consent is documented on a written consent form and signed by the subject.
A written copy should be provided to the subject. The full form may be read to the subject by the
researcher.
Documented Oral Consent
Sometimes consent is collected orally. In this case, the researcher provides the required informed
consent information to the potential subject verbally, and provides a written short form version of the
consent form that is signed by the subject. In this case, a witness must be present for the verbal
presentation, and must also sign the short form. A copy of the full summary of informed consent
information must be provided to the subject.
Waiver of Documented Consent
The NYC DOE IRB may waive the requirement for the researcher to obtain a signed informed consent
form in certain cases, if:
• The only record linking the subject and the research would be the informed consent form and
the principal risk would be potential harm resulting from a breach of confidentiality. Each
subject (or legally authorized representative) will be asked whether the subject wants
documentation linking the subject with the research, and the subject’s wishes will govern;
• The research presents no more than minimal risk of harm to subjects and involves no
procedures for which written consent is normally required outside of the research context; or
• The subjects or legally authorized representatives are members of a distinct cultural group or
community in which signing forms is not the norm, that the research presents no more than
minimal risk of harm to subjects and provided there is an appropriate alternative mechanism for
documenting that informed consent was obtained.
In cases in which the documentation requirement is waived, the IRB may require the investigator to
provide subjects or legally authorized representatives with a written statement regarding the research.
49
Electronic Consent
Researchers may choose to collect informed consent electronically. For example, a study may include an
online survey, and the first page of the survey may be the consent/assent page. If you plan to collect
consent electronically, consider the following.
If you are collecting documented informed consent:
• Explain how you will provide access to all the informed consent information (link to a PDF?
Download a doc?)
• Clarify how you will collect subject names and signatures on the electronic platform (separate
page? Virtual signature? Type in name?)
• Describe how consent information will be stored (in survey platform, other place)
• Explain if subjects’ consent name/signature will be connected to other data collected for
research (will consent info be connected to research data collection or not)
If you are getting informed consent, but requesting a waiver of documentation of consent:
• Explain how you will provide the opportunity to consent (such as, a statement on the first page
of a survey and a checkbox saying, “I consent to participate”)
• Explain how you will provide access to all the informed consent information
Adult Consent
Adult consent procedures or a request for a waiver are required for any research activities conducted
with adults.
Parental Consent
Parental consent procedures or a request for a waiver are required for any research activities conducted
with NYC DOE students, including students over 18. The parental consent form should be written to
describe what the child will do as part of the research.
The parental consent form is different from the adult consent form. If a parent will also be a research
participant themselves, they will need to sign a separate adult consent form for their own participation.
If you intend to access student administrative data and you do not qualify for a FERPA exception, you
will need to collect parental consent to access this data. Ensure the consent form describes the
administrative data in detail. Include a separate signature line for parents to consent to the data
sharing.
If you will be using a FERPA exception to access student administrative data, do not include a signature
line in the parental consent form, since this will override any FERPA exception. Instead, include an
explanation that you will access student administrative data for this study through a FERPA exception,
and that you are not requesting parental consent to access the data.
DOE Students over 18
For research participants who are DOE students aged 18-21, the NYC DOE IRB requires both parental
consent (signed by parent/guardian) and adult consent (signed by adult student).
50
Child Assent
The NYC DOE IRB requires assent procedures or a request for a waiver for any participants under the age
of 18. The assent procedures must explain what happens if a child chooses not to participate. Parents
may not administer assent for their children. Teachers may not administer assent for students, unless
they are part of the research study team. The proposal must provide a clear explanation of how the
assent process will occur with children, including who will administer assent, how any questions will be
addressed, and how children will provide assent.
Informational Letters
For some research procedures, a waiver of parental consent is granted, but the NYC DOE IRB requires
researchers to instead distribute an information letter to parents. The information letter can include the
same sections as the consent form, but would not include a signature line, or would include an opt out
signature line, if required.
Consent forms for multiple research activities/populations
You may combine multiple research activities for the same participant population in a single
consent/assent form, if appropriate given the study design and timeline.
If the study involves DOE students who are both under and over 18 years old, you may combine the
child assent and adult consent forms. Please include two signature lines on the form – one for students
17 and under to provide assent, and one for students 18 and older to provide consent. In this case, the
form must include all of the required elements for adult consent. This may not be an appropriate option
if the form is also being used to collect assent from young students.
Parental consent for unaccompanied minors
While rare, sometimes unaccompanied minors are targeted for research participation. In this case,
obtaining parental consent is difficult or impossible.
The federal guidance states:
“In addition to the provisions for waiver contained in §46.116 of subpart A, if the IRB determines that a
research protocol is designed for conditions or for a subject population for which parental or guardian
permission is not a reasonable requirement to protect the subjects (for example, neglected or abused
children), it may waive the consent requirements in Subpart A of this part and paragraph (b) of this
section, provided an appropriate mechanism for protecting the children who will participate as subjects
in the research is substituted, and provided further that the waiver is not inconsistent with federal, state,
or local law. The choice of an appropriate mechanism would depend upon the nature and purpose of the
activities described in the protocol, the risk and anticipated benefit to the research subjects, and their
age, maturity, status, and condition.”
In these cases where it does not make sense to collect parental consent, the NYC DOE IRB may waive the
requirement, as long as appropriate considerations and protections are in place. These considerations
and protections might be, ensuring the nature of the research activities are low risk, considering the
child’s maturity to provide assent, or ensuring a trained assent monitor is in place for any child assent

51
process where there is no parental consent. The basic idea is to ensure children are not coerced into
participation, are able to make an informed decision about participation, feel empowered to consent or
decline, feel comfortable reporting concerns, and are adequately protected from harm throughout the
research.
In the research proposal, the submission would request a waiver of parental consent for these students
specifically, and then explain how the researcher would protect those children throughout the process.
5.3 Interactions with Research Subjects
5.3.1 Asking children sensitive questions
The NYC DOE IRB pays careful attention to protecting our students. The following types of questions are
considered sensitive, and are typically reviewed with extra scrutiny by the Board. If research intends to
ask about these sensitive topics, researchers must provide substantial justification for why it is
necessary, and how they will be protecting this information. In many cases, asking about these topics is
not allowed.
The NYC DOE IRB typically does not allow these types of sensitive questions:
• Place of birth (of child, or family members)
• Immigration status
• Address
• Questions with answers that could lead to potential incrimination (such as, asking about illegal
behaviors)
• Questions with answers that could trigger mandated reporting (such as, asking about violence in
the home)
The following questions are allowed, but with the following recommendations:
• Gender (recommend having inclusive, non-binary options)
• Parent/guardian information (recommend having inclusive, non-binary options, instead of
“Mother” and “Father”)
5.3.2 Procedures for conducting school or classroom observations -- Updated May 2024
School Observations
Requests to conduct school observations (such as school walkthroughs, building observations, etc.)
require NYC DOE IRB approval, and are considered on a case-by-case basis. IRB submissions must include
a clear justification for the school observation and a detailed protocol or description of what will be
observed. In some cases, it may be necessary to provide a parent/student information letter to inform
students and families about the presence of researchers in the school conducting observations.
Recording of school observations is rarely approved. After DOE approval, the researcher must work with

52
the school principal to determine additional requirements for a school walkthrough (such as, who would
escort the researcher, where they are allowed to go, etc.).
Classroom Observations and Consent
In reviews and determinations, the DOE IRB has typically considered any observations in schools
(including classrooms, hallways, meetings) as private and subject to requirements for consent. There
may be some specific situations that would be considered public (such as public meetings or events
occurring in a school building) where we might not require active consent. That said, the DOE IRB often
applies additional requirements depending on the details of the study.
The DOE IRB usually takes a fairly conservative approach to observations happening in schools, and we
typically require some sort of consent or notification for observations.
Typically, for classroom observations where the students are not the subjects of the observation, we
require active teacher consent, approve a waiver of parental consent for students, and require an
informational letter to be sent home to parents informing them of the observation. If the observation
entails additional sensitivity or risk (perhaps a sensitive subject area, or an especially vulnerable
population), we ask the researcher to send an opt out informational letter to parents so they can opt
their child out of the observation if they wish.
For classroom observations where students are the focus, in addition to teacher consent, we require
active parental consent and student assent for all students in the classroom, since it is unrealistic for a
researcher to keep track of consented and unconsented students in a classroom observation.
As an alternative, due to the applicability of §46.117(c)(1)(ii) (that the research presents no more than
minimal risk of harm to subjects and involves no procedures for which written consent is normally
required outside of the research context), researchers may request a waiver of parental consent for
students’ participation in the classroom observations. Approval of this waiver is contingent upon the
following:
- To comply with the waiver requirements of minimal risk, the researcher must clarify how the
observation protocol will ensure no identifiable data is collected about students.
- The researcher may not interact with students in the classroom.
- The researcher must provide and distribute a parent information letter describing the classroom
observations (and include an opt out option, if applicable, as described above).
- Classroom observations may not be audio or video recorded.
If the researcher wants to audio record the observation, the DOE IRB has stricter rules. For observations
of classroom activities that are interactive (where students are expected to be talking, and where it is
likely student voices will be captured in the audio recording), we ask the researcher to either explain
how they will avoid capturing student voices in the recording, or obtain active parental consent and
student assent for all students in the classroom, along with teacher consent. If the observation will just
capture a teacher’s instruction (where it is not expected that students will be talking much), the board
may approve a waiver of parental consent with an informational letter for parents (with an opt out
option as appropriate), along with teacher consent. We rarely allow video recording of students.

53
In cases where there are co-teachers in a classroom, both teachers must give consent for classroom
observations, even if just one teacher is the focus of the study.
Consent Requirements for Classroom Observations
Consent Requirements
Type of Observation
Audio
recorded?*
Teacher
consent
Parental Consent
Student
Assent
Classroom observation of teacher
instruction only (students are not
subjects of observation)
No or Yes
Required
Eligible for waiver
Info letter required
Optional
Classroom observation of teacher
instruction and student behaviors
(student words/actions will be
captured in observation notes or
recordings)
No
Required
Eligible for waiver, but
must prove minimal risk
Info letter required
Optional
Yes
Required
Required
Required
*Video recording is rarely approved, and only approved under special circumstances.
5.3.3 Conducting Focus Groups -- Updated May 2024
The NYC DOE IRB recommends using the following procedures when conducting focus groups:
• The focus group protocol should begin by setting expectations or norms for participants,
including:
o Do not share specific names or identifying information that could identify any students
or other non-participants.
o Do not share specific details that would break the confidentiality of students or other
non-participants.
o Do should not take screenshots or record the focus group activity.
o Information shared in this focus group is meant for the participants and researchers
only. Please do not share information from the focus group conversation with others
who were not part of the group.
o Participate in the focus group from a quiet and private place. If you cannot be in a
private space, please wear headphones to avoid unintentionally sharing information
with others in the space who are not a part of the focus group.
• Prior to starting any recording, ask for additional verbal consent for recording focus groups,
even if you already collected documented consent that included consent for recording.
5.3.4 Conducting Interviews -- Updated May 2024
Prior to starting any recording, ask for additional verbal consent for recording interviews, even if you
already collected documented consent that included consent for recording.

54
5.3.5 Collecting artifacts
Some studies request access to artifacts, such as student work, teacher lesson plans, or agendas from
professional learning sessions. Student work is protected under FERPA, so would require parental
permission or a FERPA exception to access for research purposes. Requests to collect artifacts containing
identifiable information are reviewed on a case-by-case basis.
Requests to collect artifacts that do not include identifiable information or student work are typically
allowed (for example, teacher lesson plans, professional development agenda), but must be explained in
the submission. This explanation must include specific details of what would be collected and how it
would be used or analyzed to answer the research questions.
5.3.6 Collecting data from educational applications -- Updated May 2024
Studies that intend to use data collected from students or school staff from third party applications or
websites used in the classroom must include a detailed description of this data in the NYC DOE IRB
submission form. This data collection may include:
• Data entered into an app for the app to function (username, grade level)
• Data collected by the app through students' interaction (usage time, progress, screen capture)
• Assessments conducted in the app
• Data collected for app functionality (responsive lessons)
A detailed description of this data use must be included in the IRB application, even if it is data the
submitter has access to already.
Please note, all vendors of third-party software are required to complete the DOE's compliance process
and OTI's cloud review process before conducting business with the DOE. See more information here:
Data Privacy and Security Compliance Process (nyced.org)
5.3.7 Collecting any medical or biometric data
With rare exceptions, the DOE does not allow collection of medical or biometrics data from students,
including blood pressure, finger stick blood tests, fit bits, pedometers, etc. In rare cases, the NYC DOE
IRB has approved collection of student height and weight with adequate justification, parent consent,
and explicit considerations for student privacy.
5.3.8 Recording (audio, video, photography)
Recording may include audio, video, photographic or other recording of research subjects for the
purposes of data collection for research or evaluation. Recording the voice and/or image of an individual
creates a type of record that requires unique handling and storage, particularly if the content may be
considered sensitive. As with all research procedures, the dignity of human subjects should be
respected. Therefore, only what is necessary for the purpose of the study should be recorded. Research
subjects must be informed prospectively that such recording will occur, and be provided with
information about the storage, confidentiality, and future use of the resulting tape or digital record.
55
Requirements for Submissions Requesting Recording
If a research protocol involves the recording of research subjects, the study team must include the
following elements in their protocol and associated materials for IRB review:
• The type of recording (e.g. video, photo, audio, etc.), that will be utilized.
• Specific identifiers that will be recorded, either intentionally or not (e.g., partial facial features,
full facial features, subject’s name).
• People who will have access to the recording(s).
• Mechanisms in place to protect the privacy of the person(s) being recorded (e.g. blurring faces,
explicitly not recording full facial features or other direct or indirect identifiers, not audio-
recording names or other identifying information, etc.).
• Clear indication of when the recording(s) will be destroyed or that recording(s) will be kept
indefinitely/specified duration.
• Use(s) of the recording(s), including educational or commercial purposes, analysis by the
research team, or future unspecified use or sharing.
• Compensation, if any, to subjects for allowing themselves to be taped.
NYC DOE IRB Policies on Recording
The NYC DOE IRB applies additional consideration to proposed recording of children/minors, parents,
DOE staff, or recordings in a school.
• Video recording for research purposes is not generally permitted, with some very limited
exceptions that are made on a case-by-case basis.
• Audio recording is permitted, but requires explicit documented consent from all adult
participants, documented (age permitting) assent from the child, and documented parental
permission. Please see the section above on classroom observations for consent requirements
for classroom observations.
• Photographing for research purposes is considered on a case-by-case basis. Photographs that
include full facial features are not generally permitted. Photographs of student work require
prospective FERPA authorization through parental permission, or qualification for a FERPA
exception.
• Recording virtual interactions (such as over Zoom or Microsoft Teams) is allowed, following the
same consent requirements and policies detailed above. Additionally, submissions must explain
the steps taken to ensure the protection of recordings. When recording using a virtual
telecommunication tool or platform, the submission will need to explain where the recording
would be stored and if it would stored/owned/used by the tool/platform. The NYC DOE IRB
recommends using institutionally licensed versions of tools/platforms since they usually have
more robust data security protection measures in place.
In general, recordings should be protected using the same data security procedures as other data
collected, including secure storage using password protection and encryption, and secure data
transmission. See Data Security section for more information [coming soon].

56
Include Recording in the Consent Form
If recording is an integral part of the research and not an optional procedure, a separate informed
consent document is not required. However, documentation of the considerations listed above must be
included within the body of the informed consent document for the overall study. It is important that
this information be clearly stated, preferably preceded by a heading, so that it is clear to the subject that
a recording will be made.
If the recording is not required as part of the research procedures, then the consent document must
include a specific statement indicating that participation in the research study is not contingent upon
agreeing to be recorded. A separate consent signature for permission to record will be necessary and
must be included within the body of the informed consent document. The DOE consent form templates
include this separate recording signature section for reference.
Additional Circumstances
• Some studies request that teachers record themselves teaching and submit this recording to
researchers. These requests are reviewed on a case-by-case basis. In reviewing these requests,
the Board looks at if teacher would provide consent, the burden on teachers, if student voices
would be captured in the recording, the sensitivity of the content, if the recording would be
video or just audio, and the details of the described procedures for recording and submitting
(where would the recording device be located, what platform would be used for teachers to
submit recordings, etc.).
Transcription services
[Coming soon]
5.3.9 Compensation for research -- Updated May 2024
The NYC DOE has specific policies pertaining to study subject compensation.
DOE Personnel Compensation
• NYC Conflicts of Interest Rules prohibit teachers and other NYC DOE staff from receiving
compensation in exchange for participation in research studies. Compensation/gifts that benefit
the entire school may be donated directly to the school.
• Researchers may no longer contribute to a specific teacher or classroom. Contributions must be
made to the school.
• Compensation can be in the form of gift cards given to the school or funds deposited into the
school’s bank account. Compensation for research must be used in alignment with the school’s
standard operating procedures. Any compensation of $10,000 or more must go through the NYC
Fund for Public Schools.
• Anything purchased by the school using funds received through research compensation belongs
to the school. Individual school staff who participated in the research activities cannot be
directly compensated, and items purchased using research compensation funds do not belong
to any individual staff members.
57
• Exceptions to this policy are extremely rare and securing a waiver can be a lengthy process
requiring a thorough review by the DOE Ethics Officer, the NYC Conflicts of Interest Board and,
ultimately, the Office of the Schools Chancellor.
• Some DOE programs, especially Universal Pre-K programs, are located in Community-Based
Organizations (CBOs). CBO staff are not members of the United Federation of Teachers and are
not salaried DOE employees. Researchers conducting studies in CBOs should check with the
CBO’s leadership to determine whether staff in their facility can be compensated for
participating in research.
Quid Pro Quo Compensation
• Compensation cannot be offered to schools on a quid pro quo basis, meaning the amount of
compensation cannot be tied to the amount of research participation by the school. Quid pro
quo compensation has the potential to compromise the voluntariness of the schools or
individual research subjects’ participation in the study.
• The following examples would be considered quid pro quo compensation and would not be
allowed:
o Basing the amount of a school donation on the number of teachers who agree to
participate in the research.
o Donating more to a school if teachers complete more data collection surveys.
Student Compensation
• Gifts may be given to students for their participation. For elementary school students, stickers,
pens, or gift cards not to exceed $15 is allowed. For middle and high school students, the value
of the incentive should not exceed $25. Amounts beyond this could be coercive. Requests to
compensate more than these amounts are reviewed on a case-by-case basis.
Parent Compensation
• Compensation for parents/guardians can be based on the number of hours required for their
participation in the research (for interviews, and focus groups) or per completed research
activity (e.g., survey, interview) where the amount of time involved might vary depending on the
type and number of research activities requested of parents. Compensation should not involve a
sum of money that would be perceived as coercive (as determined during IRB review).
Other notes on compensation
• Compensation may be monetary or non-monetary in nature. Reimbursement for out-of-pocket
expenses is not considered compensation and, if applicable, should be described separately in
the protocol and consent document.
• Research proposals will need to describe when and where study subjects will be compensated
and detail the mechanisms that will be in place to ensure study subject privacy when
distributing compensation.
• Research proposals may need to describe how participants will be compensated if they
withdraw from the research.

58
o For example, explain if study subjects will be compensated for completing half of the
proposed research procedures and specify how much they will receive for partial or
incomplete participation.
• Raffles are not appropriate methods of compensation.
How the Board reviews compensation procedures
The NYC DOE IRB considers the following when reviewing plans for compensating individual participants
who are not DOE employees:
• Compensation must be fair, given the amount of time and effort participants must give to the
research activities. Submissions may provide justification for the proposed amount of
compensation.
• If the amount of compensation varies, the method for calculating the amount of compensation
must be explained in the protocol and explained to participants.
• The timeframe and amount of compensation must be made clear to participants. If
compensation is connected to completion of research activities, or distributed in phases, this
must be clearly explained.
The NYC DOE IRB considers the following when reviewing plans for compensating schools:
• Compensation is distributed directly to the school.
• Compensation is not based on the amount of research participation in the school (such as,
compensation based on the number of teachers who fill out a survey).
• Compensation may be based on the number of potential participants.
• The amount of school compensation must be clearly explained in the Principal Permission
letter.
• If the amount of compensation varies, the method for calculating the amount of compensation
must be explained in the protocol and explained to school principals.
Compensation Procedures
In the submission to the NYC DOE IRB, the researcher must clearly explain how any compensation will be
distributed. If compensation will be distributed in person, this process must be explained. If
compensation will be distributed electronically, this process must be explained, and the researcher must
explain how they will collect any participant contact information, if that contact information will be
connected with any research data, how the researcher will keep contact information collected for
research separate from any research data (if applicable), and if they will keep the contact information
for any other purposes.
5.3.10 Deception or Non-Disclosure -- Updated May 2024
Some research studies use deception or non-disclosure as part of the research procedures. The NYC DOE
IRB rarely approves of deception in a research study in our jurisdiction. Use of deception in research
with students is not likely to be approved. Certain types of deception in research with adults might be
used when there is no other way to obtain unbiased data from respondents and when the benefits far
outweigh the risks.
59
Use of deception requires a detailed debriefing protocol to be used with respondents at the conclusion
of their participation in the research, when they are informed that deception was used. A debriefing
script must be attached for IRB review as a study instrument.
Deception relating to guarantees of confidentiality or anonymity for research participants is never
permitted. It is up to the discretion of the NYC DOE IRB to determine whether deception or non-
disclosure is appropriate.
60
6. Appendices
6.1 Definitions
• Human subjects: A living individual about whom an investigator (whether professional or
student) conducting research (i) Obtains information or biospecimens through intervention or
interaction with the individual, and uses, studies, or analyzes the information or
biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private
information or identifiable biospecimens.
• Research: A systematic investigation, including research development, testing, and evaluation,
designed to develop or contribute to generalizable knowledge.
• NYC DOE schools or affiliates: This includes any students, school-based staff, non-school-based
staff, parents recruited through the schools, or other NYC DOE affiliates as determined by the
NYC DOE IRB.
6.2 CITI Training Requirements
NYC DOE CITI Course Requirement
• Social & Behavioral Research (ID: 184110)
• Required Modules: 26
o Conflicts of Interest in Human Subjects Research (ID: 17464)
o Students in Research (ID: 1321)
o Unanticipated Problems and Reporting Requirements in Social and Behavioral Research
(ID: 14928)
o History and Ethical Principles - SBE (ID: 490)
o Defining Research with Human Subjects - SBE (ID: 491)
o The Federal Regulations - SBE (ID: 502)
o Assessing Risk - SBE (ID: 503)
o Informed Consent - SBE (ID: 504)
o Privacy and Confidentiality - SBE (ID: 505)
o Vulnerable Subjects - Research Involving Workers/Employees (ID: 483)
o Populations in Research Requiring Additional Considerations and/or Protections (ID:
16680)
o Research with Children - SBE (ID: 507)
o Research in Public Elementary and Secondary Schools - SBE (ID: 508)
o Internet-Based Research - SBE (ID: 510)
o Consent and Subject Recruitment Challenges: Remuneration (ID: 16881)
o Consent Tools Used by Researchers (ID: 16944)
o Introduction To Community-Engaged Research (CEnR) (ID: 16994)
o Introduction to Community-Based Participatory Research (CBPR) (ID: 16995)
o Ethical and Practical Considerations in Community-Engaged Research (CEnR) (ID: 16996)
o Consent in the 21st Century (ID: 17060)
o Consent with Subjects Who Do Not Speak English (ID: 17260)
o Consent and Cultural Competence (ID: 17263)
o Belmont Report and Its Principles (ID: 1127)
61
o Research with Persons who are Socially or Economically Disadvantaged (ID: 16539)
o Illegal Activities or Undocumented Status in Human Research (ID: 16656)
o Cultural Competence in Research (ID: 15166)
• Optional Modules
o Humanitarian Use Devices (HUDs) (ID: 16306)
o Research with Older Adults (ID: 16502)
o Research and HIPAA Privacy Protections (ID: 14)
o Research with Subjects with Physical Disabilities & Impairments (ID: 16657)
o Research Involving Subjects at the End-of-Life (ID: 16658)
o Ethical and Appropriate Uses of Administrative Data for Research and Evaluation (ID:
19826)
o The IRB Administrator's Responsibilities (ID: 13813)
o Gender and Sexuality Diversity (GSD) in Human Research (ID: 16556)
o Research with Critically Ill Subjects (ID: 16592)
o Research with Decisionally Impaired Subjects (ID: 16610)
o External IRB Review (ID: 16711)
o Phase I Research: Understanding Phase I Research (ID: 16873)
o Phase I Research: Protecting Phase I Subjects (ID: 16874)
o Informed Consent and Incidental Findings in Research with Human Subjects (ID: 17342)
o Overview of the Clinical Trial Agreement (CTA) (ID: 17356)
o Understanding the Terms of the Clinical Trial Agreement (CTA) (ID: 17357)
o Role of the Researcher and Site in Managing the Clinical Trial Agreement (CTA) (ID:
17358)
o Clinical Trial Agreement (CTA) Negotiation for Researchers and Sites (ID: 17359)
o Disaster and Conflict Research, Part 1: PI Responsibilities (ID: 17384)
o Disaster and Conflict Research, Part 2: Best Practices and Recommendations (ID: 17385)
o Single Institutional Review Board (sIRB) Use and Administration: When Relying on a sIRB
(ID: 17387)
o Single Institutional Review Board (sIRB) Use and Administration: When Serving as a sIRB
of Record (ID: 17388)
o Single Institutional Review Board (sIRB) Use and Administration: Authorization
Agreements (ID: 17392)
o Consent and Biobanks and Associated Databases (ID: 17254)
o Consent and Subject Recruitment Challenges: Therapeutic Misconception (ID: 17259)
o The IRB Member Module - 'What Every New IRB Member Needs to Know' (ID: 816)
o International Research - SBE (ID: 509)
o I Have Agreed to be an IRB Community Member. Now What? (ID: 13018)
o Hot Topics (ID: 487)
o Research with Prisoners - SBE (ID: 506)

62
6.3 Guidance for DOE students completing research -- Updated May 2024
While the NYC DOE IRB does not review or approve research conducted by DOE students, the DOE IRB
has developed basic guidelines to assist schools overseeing student research and data collection. These
guidelines embody many of the federal regulations governing research with human subjects as well as
policies and procedures that reflect the jurisdictional concerns of the NYC DOE. These guidelines are
recommendations, and the DOE IRB does not review or approve research conducted by DOE students.
• Students may conduct research with other students in their own school or at other schools,
provided that they submit a research proposal to their teacher outlining:
o the research topic
o design/methodology
o risks/benefits
o measures that will be taken to protect the privacy and confidentiality of research
participants
o who will have access to the data
o how the research findings will be used
• Students conducting research with other students must make it clear that participation is
voluntary, and participants can withdraw at any time or chose not to answer any questions.
• Students conducting classroom observations should obtain the consent of the classroom
teacher(s). Data collection should not distract from classroom instructional time.
• Survey instruments should include a check box for students to indicate they have agreed to be
surveyed, thus protecting the anonymity of respondents. When a research project involves a
pre- and post-test, a list of the names of respondents to the pre-test and the research ID
numbers assigned to them should be stored in a secure location. After the post-test, all names
should be removed from survey instruments and replaced with research ID numbers.
• For projects including interviews or focus groups, student researchers should provide an assent
form (for students 17 and under) or consent form (for students 18 and over) to be signed and
dated by the research participant.
• On any transcripts of interview or focus groups, names of student participants should be
replaced with research ID numbers to ensure the privacy and confidentiality of participants.
• Interviews and focus groups may be audiotaped with the permission of participants.
• Videotaping public school students for research purposes is prohibited by DOE policy.
• All data collected from research participants should be reported anonymously.
• Student researchers should refrain from collecting information that might expose study
participants to (1) psychological risk such as discomfort, embarrassment, worry or anxiety; (2)
social risk such as damage to reputation; (3) risk of breach of confidentiality or anonymity.
o Examples of this type of information would be religious affiliation, sexual identity and
behaviors, use of alcohol, drugs, illegal behaviors, and other information that might be
self-incriminating.
• Student researchers should not use deception in recruiting other students to participate in their
research. The purpose of the research should be clearly stated.
• Teachers and students should ensure that all hard copy and electronic data are securely stored
to prevent unauthorized access, disclosure, or loss. Hard copy records should be stored in a
manner that limits access to only authorized individuals. For example, filing cabinets/areas
should be locked and placed in secured/locked rooms.
