
230
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
ABSTRACT
Articial intelligence (AI) aims to mimic human cognitive
functions. It is bringing a paradigm shift to healthcare,
powered by increasing availability of healthcare data and
rapid progress of analytics techniques. We survey the
current status of AI applications in healthcare and discuss
its future. AI can be applied to various types of healthcare
data (structured and unstructured). Popular AI techniques
include machine learning methods for structured data,
such as the classical support vector machine and neural
network, and the modern deep learning, as well as
natural language processing for unstructured data. Major
disease areas that use AI tools include cancer, neurology
and cardiology. We then review in more details the AI
applications in stroke, in the three major areas of early
detection and diagnosis, treatment, as well as outcome
prediction and prognosis evaluation. We conclude with
discussion about pioneer AI systems, such as IBM Watson,
and hurdles for real-life deployment of AI.
OVERVIEW OF THE MEDICAL ARTIFICIAL
INTELLIGENCE (AI) RESEARCH
Recently AI techniques have sent vast waves
across healthcare, even fuelling an active
discussion of whether AI doctors will eventu-
ally replace human physicians in the future.
We believe that human physicians will not
be replaced by machines in the foreseeable
future, but AI can definitely assist physicians to
make better clinical decisions or even replace
human judgement in certain functional areas
of healthcare (eg, radiology). The increasing
availability of healthcare data and rapid devel-
opment of big data analytic methods has
made possible the recent successful applica-
tions of AI in healthcare. Guided by relevant
clinical questions, powerful AI techniques can
unlock clinically relevant information hidden
in the massive amount of data, which in turn
can assist clinical decision making.
1–3
In this article, we survey the current status
of AI in healthcare, as well as discuss its future.
We first briefly review four relevant aspects
from medical investigators’ perspectives:
1. motivations of applying AI in healthcare
2. data types that have be analysed by AI sys-
tems
3. mechanisms that enable AI systems to gen-
erate clinical meaningful results
4. disease types that the AI communities are
currently tackling.
Motivation
The advantages of AI have been extensively
discussed in the medical literature.
3–5
AI
can use sophisticated algorithms to ‘learn’
features from a large volume of healthcare
data, and then use the obtained insights to
assist clinical practice. It can also be equipped
with learning and self-correcting abilities to
improve its accuracy based on feedback. An
AI system can assist physicians by providing
up-to-date medical information from jour-
nals, textbooks and clinical practices to
inform proper patient care.
6
In addition, an
AI system can help to reduce diagnostic and
therapeutic errors that are inevitable in the
human clinical practice.
3 4 6–10
Moreover, an
AI system extracts useful information from
a large patient population to assist making
real-time inferences for health risk alert and
health outcome prediction.
11
Healthcare data
Before AI systems can be deployed in health-
care applications, they need to be ‘trained’
through data that are generated from clin-
ical activities, such as screening, diagnosis,
treatment assignment and so on, so that they
can learn similar groups of subjects, associa-
tions between subject features and outcomes
of interest. These clinical data often exist in
but not limited to the form of demographics,
medical notes, electronic recordings from
medical devices, physical examinations and
clinical laboratory and images.
12
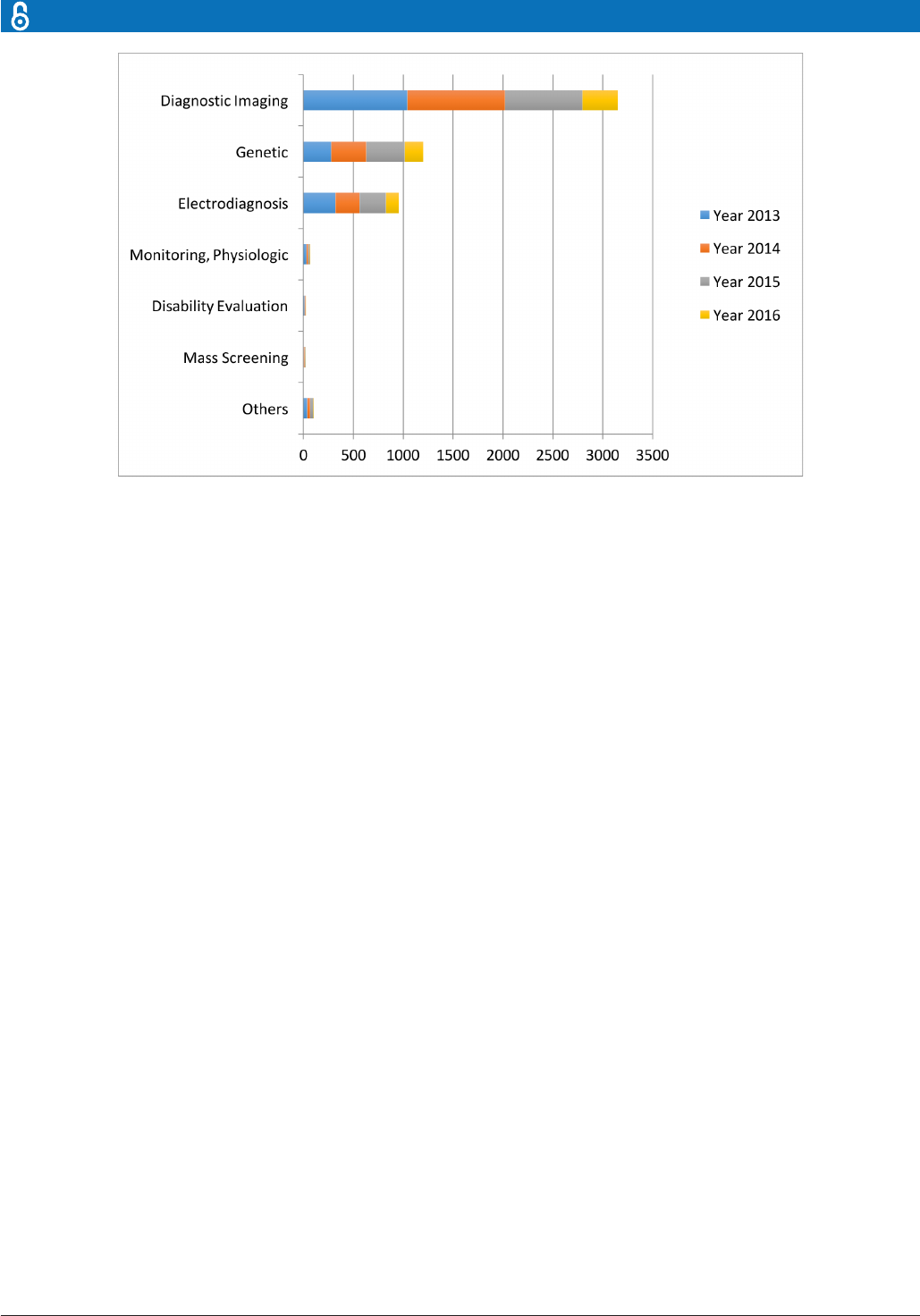
Specifically, in the diagnosis stage, a substan-
tial proportion of the AI literature analyses
data from diagnosis imaging, genetic testing
and electrodiagnosis (figure 1). For example,
Jha and Topol urged radiologists to adopt
AI technologies when analysing diagnostic
images that contain vast data information.
13
Li et al studied the uses of abnormal genetic
Articial intelligence in healthcare: past,
present and future
Fei Jiang,
1
Yong Jiang,
2
Hui Zhi,
3
Yi Dong,
4
Hao Li,
5
Sufeng Ma,
6
Yilong Wang,
7
Qiang Dong,
4
Haipeng Shen,
8
Yongjun Wang
9
1
Department of Statistics and
Actuarial Sciences, University of
Hong Kong, Hong Kong, China
2
Department of Neurology,
Beijing Tiantan Hospital, Capital
Medical University, Beijing,
China
3
Biostatistics and Clinical
Research Methodology Unit,
University of Hong Kong Li Ka
Shing Faculty of Medicine, Hong
Kong, China
4
Department of Neurology,
Huashan Hospital, Fudan
University, Shanghai, China
5
China National Clinical
Research Center for
Neurological Diseases, Beijing,
China
6
DotHealth, Shanghai, China
7
Department of Neurology,
Tiantan Clinical Trial and
Research Center for Stroke,
Beijing, China
8
Faculty of Business and
Economics, University of Hong
Kong, Hong Kong, China
9
Department of Neurology,
Beijing Tiantan Hospital, Beijing,
China
Correspondence to
Prof Yongjun Wang;
yongjunwang1962@ gmail. com
To cite: JiangF, JiangY, ZhiH,
etal. Articial intelligence in
healthcare: past, present and
future. Stroke and Vascular
Neurology 2017;2: e000101.
doi:10.1136/svn-2017-000101
Received 12 June 2017
Accepted 14 June 2017
Published Online First
22June2017
Review
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from
231
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
expression in long non-coding RNAs to diagnose gastric
cancer.
14
Shin et al developed an electrodiagnosis support
system for localising neural injury.
15
In addition, physical examination notes and clinical
laboratory results are the other two major data sources
(figure 1). We distinguish them with image, genetic and
electrophysiological (EP) data because they contain large
portions of unstructured narrative texts, such as clin-
ical notes, that are not directly analysable. As a conse-
quence, the corresponding AI applications focus on first
converting the unstructured text to machine-understand-
able electronic medical record (EMR). For example,
Karakülah et al used AI technologies to extract pheno-
typic features from case reports to enhance the diagnosis
accuracy of the congenital anomalies.
16
AI devices
The above discussion suggests that AI devices mainly fall
into two major categories. The first category includes
machine learning (ML) techniques that analyse struc-
tured data such as imaging, genetic and EP data. In
the medical applications, the ML procedures attempt
to cluster patients’ traits, or infer the probability of the
disease outcomes.
17
The second category includes natural
language processing (NLP) methods that extract infor-
mation from unstructured data such as clinical notes/
medical journals to supplement and enrich structured
medical data. The NLP procedures target at turning texts
to machine-readable structured data, which can then be
analysed by ML techniques.
18
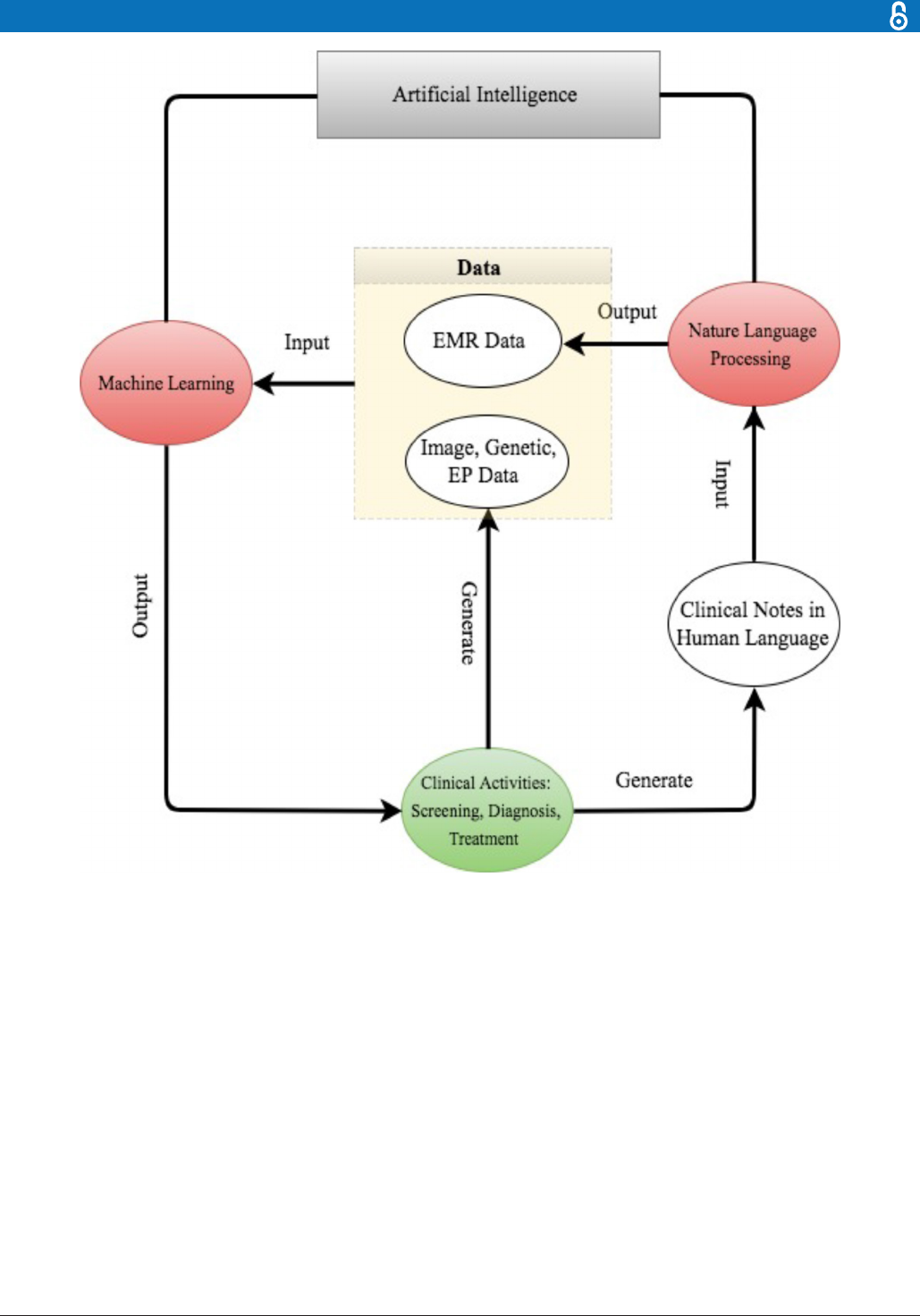
For better presentation, the flow chart in figure 2
describes the road map from clinical data generation,
through NLP data enrichment and ML data analysis, to
clinical decision making. We comment that the road map
starts and ends with clinical activities. As powerful as AI
techniques can be, they have to be motivated by clinical
problems and be applied to assist clinical practice in the
end.
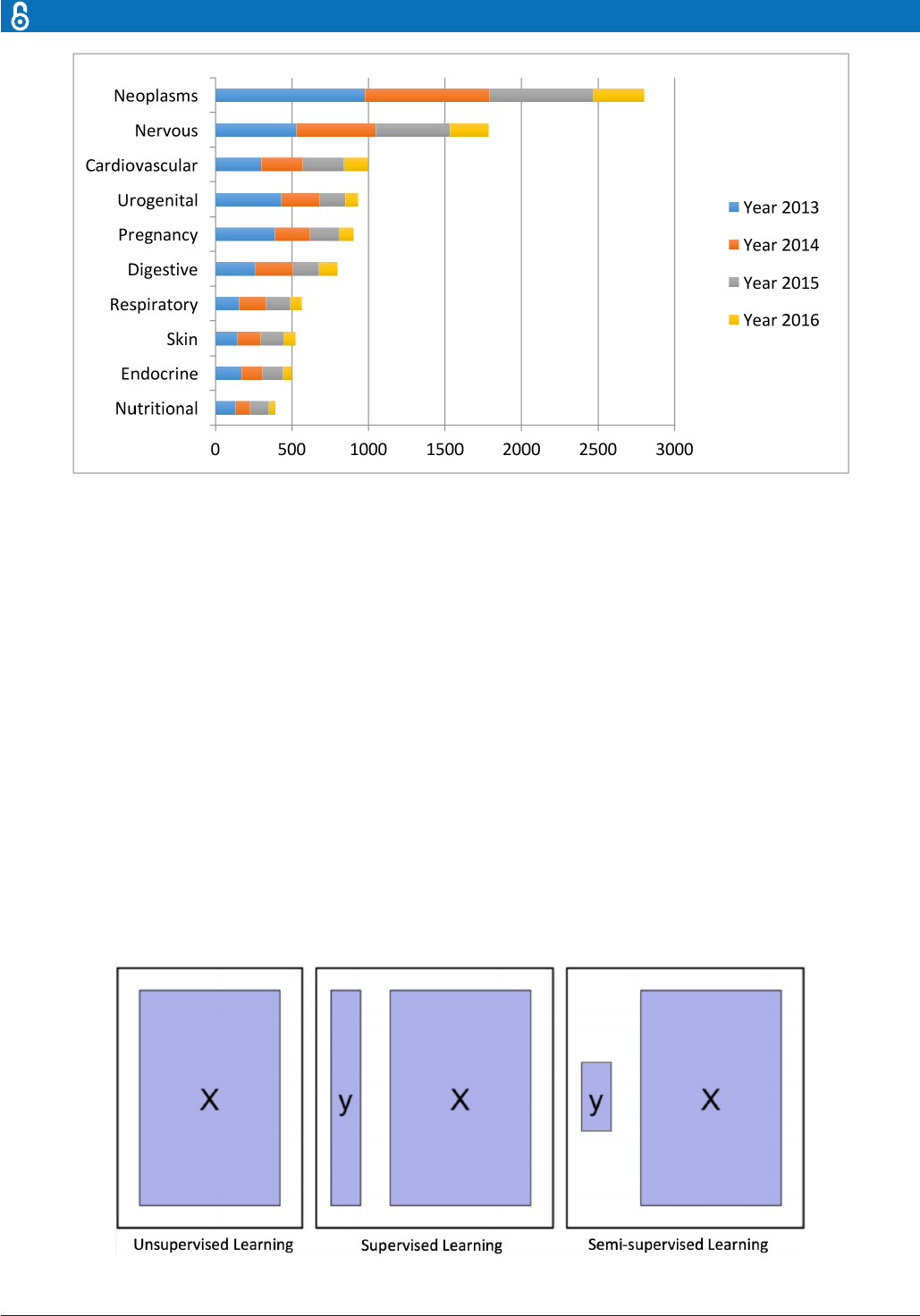
Disease focus
Despite the increasingly rich AI literature in healthcare,
the research mainly concentrates around a few disease
types: cancer, nervous system disease and cardiovascular
disease (figure 3). We discuss several examples below.
1. Cancer: Somashekhar et al demonstrated that the IBM
Watson for oncology would be a reliable AI system for
assisting the diagnosis of cancer through a double-
blinded validation study.
19
Esteva et al analysed clinical
images to identify skin cancer subtypes.
20
2. Neurology: Bouton et al developed an AI system to
restore the control of movement in patients with
quadriplegia.
21
Farina et al tested the power of an of-
fline man/machine interface that uses the discharge
timings of spinal motor neurons to control upper-limb
prostheses.
22
3. Cardiology: Dilsizian and Siegel discussed the
potential application of the AI system to diagnose
the heart disease through cardiac image.
3
Arterys
recently received clearance from the US Food and
Drug Administration (FDA) to market its Arterys
Cardio DL application, which uses AI to provide
automated, editable ventricle segmentations based on
conventional cardiac MRI images.
23
The concentration around these three diseases is not
completely unexpected. All three diseases are leading
causes of death; therefore, early diagnoses are crucial
to prevent the deterioration of patients’ health status.
Furthermore, early diagnoses can be potentially achieved
Figure 1 The data types considered in the articial intelligence articial (AI) literature. The comparison is obtained through
searching the diagnosis techniques in the AI literature on the PubMed database.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from
232
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
through improving the analysis procedures on imaging,
genetic, EP or EMR, which is the strength of the AI system.
Besides the three major diseases, AI has been applied
in other diseases as well. Two very recent examples were
Long et al, who analysed the ocular image data to diag-
nose congenital cataract disease,
24
and Gulshan et al,
who detected referable diabetic retinopathy through the
retinal fundus photographs.
25
The rest of the paper is organised as follows. In section
2, we describe popular AI devices in ML and NLP; the
ML techniques are further grouped into classical tech-
niques and the more recent deep learning. Section 3
focuses on discussing AI applications in neurology, from
the three aspects of early disease prediction and diagnosis,
treatment, outcome prediction and prognosis evaluation.
We then conclude in section 4 with some discussion about
the future of AI in healthcare.
THE AI DEVICES: MLAND NLP
In this section, we review the AI devices (or techniques)
that have been found useful in the medial applications.
We categorise them into three groups: the classical
machine learning techniques,
26
the more recent deep
learning techniques
27
and the NLP methods.
28
Classical ML
ML constructs data analytical algorithms to extract
features from data. Inputs to ML algorithms include
patient ‘traits’ and sometimes medical outcomes of
Figure 2 The road map from clinical data generation to natural language processingdata enrichment, to machine learning
data analysis, to clinical decision making.EMR,electronic medical record; EP, electrophysiological.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from
233
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
interest. A patient’s traits commonly include baseline
data, such as age, gender, disease history and so on, and
disease-specific data, such as diagnostic imaging, gene
expressions, EP test, physical examination results, clin-
ical symptoms, medication and so on. Besides the traits,
patients’ medical outcomes are often collected in clin-
ical research. These include disease indicators, patient’s
survival times and quantitative disease levels, for example,
tumour sizes. To fix ideas, we denote the jth trait of the ith
patient by X
ij
, and the outcome of interest by Y
i
.
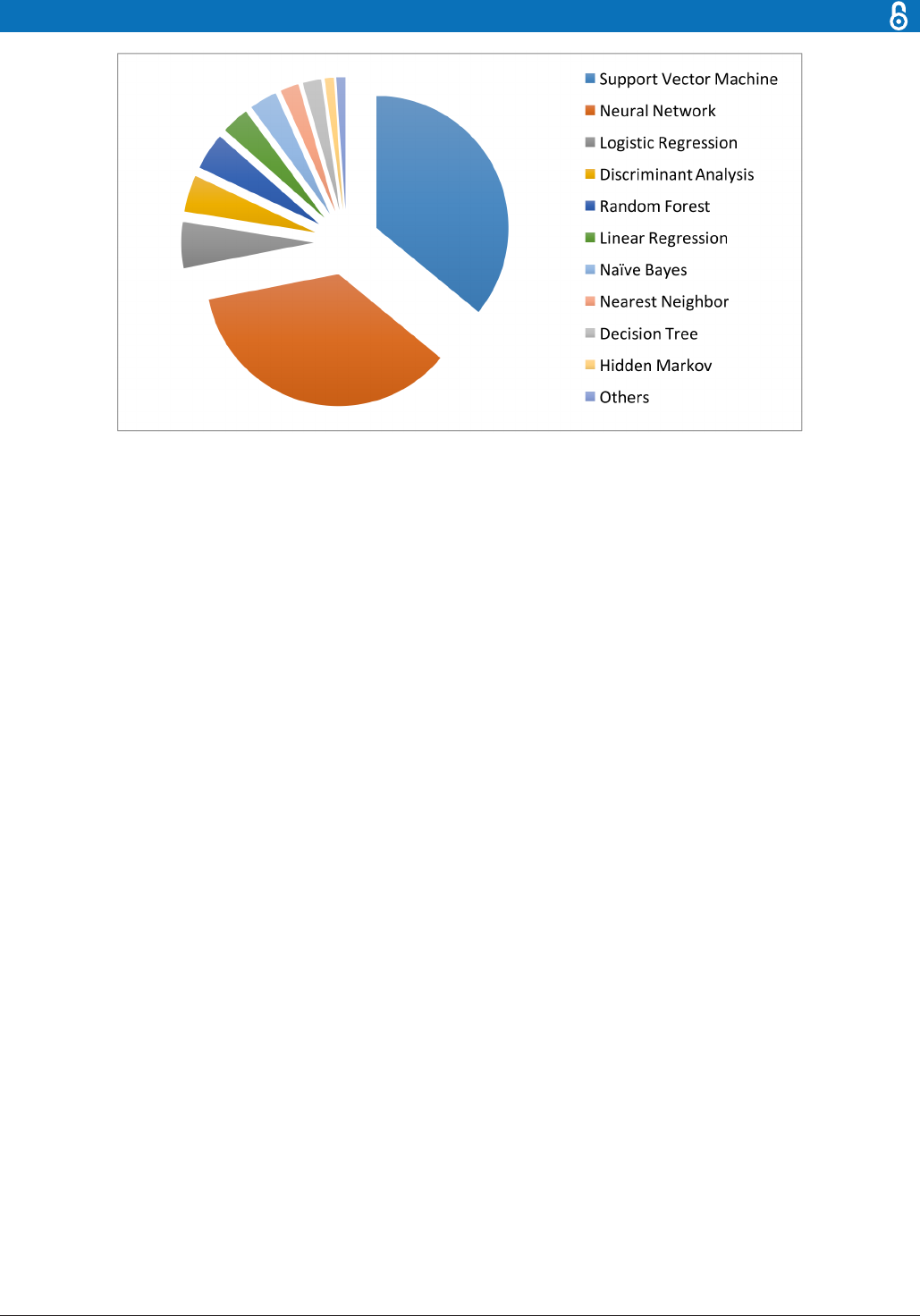
Depending on whether to incorporate the outcomes,
ML algorithms can be divided into two major categories:
unsupervised learning and supervised learning. Unsuper-
vised learning is well known for feature extraction, while
supervised learning is suitable for predictive modelling
via building some relationships between the patient traits
(as input) and the outcome of interest (as output). More
recently, semisupervised learning has been proposed
as a hybrid between unsupervised learning and super-
vised learning, which is suitable for scenarios where the
outcome is missing for certain subjects. These three types
of learning are illustrated in figure 4.
Clustering and principal component analysis (PCA)
are two major unsupervised learning methods. Clustering
groups subjects with similar traits together into clusters,
without using the outcome information. Clustering algo-
rithms output the cluster labels for the patients through
maximising and minimising the similarity of the patients
within and between the clusters. Popular clustering algo-
rithms include k-means clustering, hierarchical clustering
and Gaussian mixture clustering. PCA is mainly for dimen-
sion reduction, especially when the trait is recorded in a
large number of dimensions, such as the number of genes
in a genome-wide association study. PCA projects the data
Figure 3 The leading 10disease types considered in the articial intelligence(AI) literature. The rst vocabularies in the
disease names are displayed. The comparison isobtained through searching the disease types in the AI literature on PubMed.
Figure 4 Graphical illustration of unsupervised learning, supervised learning and semisupervised learning.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from
234
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
onto a few principal component (PC) directions, without
losing too much information about the subjects. Some-
times, one can first use PCA to reduce the dimension of
the data, and then use clustering to group the subjects.
On the other hand, supervised learning considers the
subjects’ outcomes together with their traits, and goes
through a certain training process to determine the best
outputs associated with the inputs that are closest to the
outcomes on average. Usually, the output formulations
vary with the outcomes of interest. For example, the
outcome can be the probability of getting a particular
clinical event, the expected value of a disease level or the
expected survival time.
Clearly, compared with unsupervised learning, super-
vised learning provides more clinically relevant results;
hence AI applications in healthcare most often use super-
vised learning. (Note that unsupervised learning can be
used as part of the preprocessing step to reduce dimen-
sionality or identify subgroups, which in turn makes
the follow-up supervised learning step more efficient.)
Relevant techniques include linear regression, logistic
regression, naïve Bayes, decision tree, nearest neighbour,
random forest, discriminant analysis, support vector
machine (SVM) and neural network.
27
Figure 5 displays
the popularity of the various supervised learning tech-
niques in medical applications, which clearly shows that
SVM and neural network are the most popular ones. This
remains the case when restricting to the three major data
types (image, genetic and EP), as shown in figure 6.
Below we will provide more details about the mechanisms
of SVM and neural networks, along with application exam-
ples in the cancer, neurological and cardiovascular disease
areas.
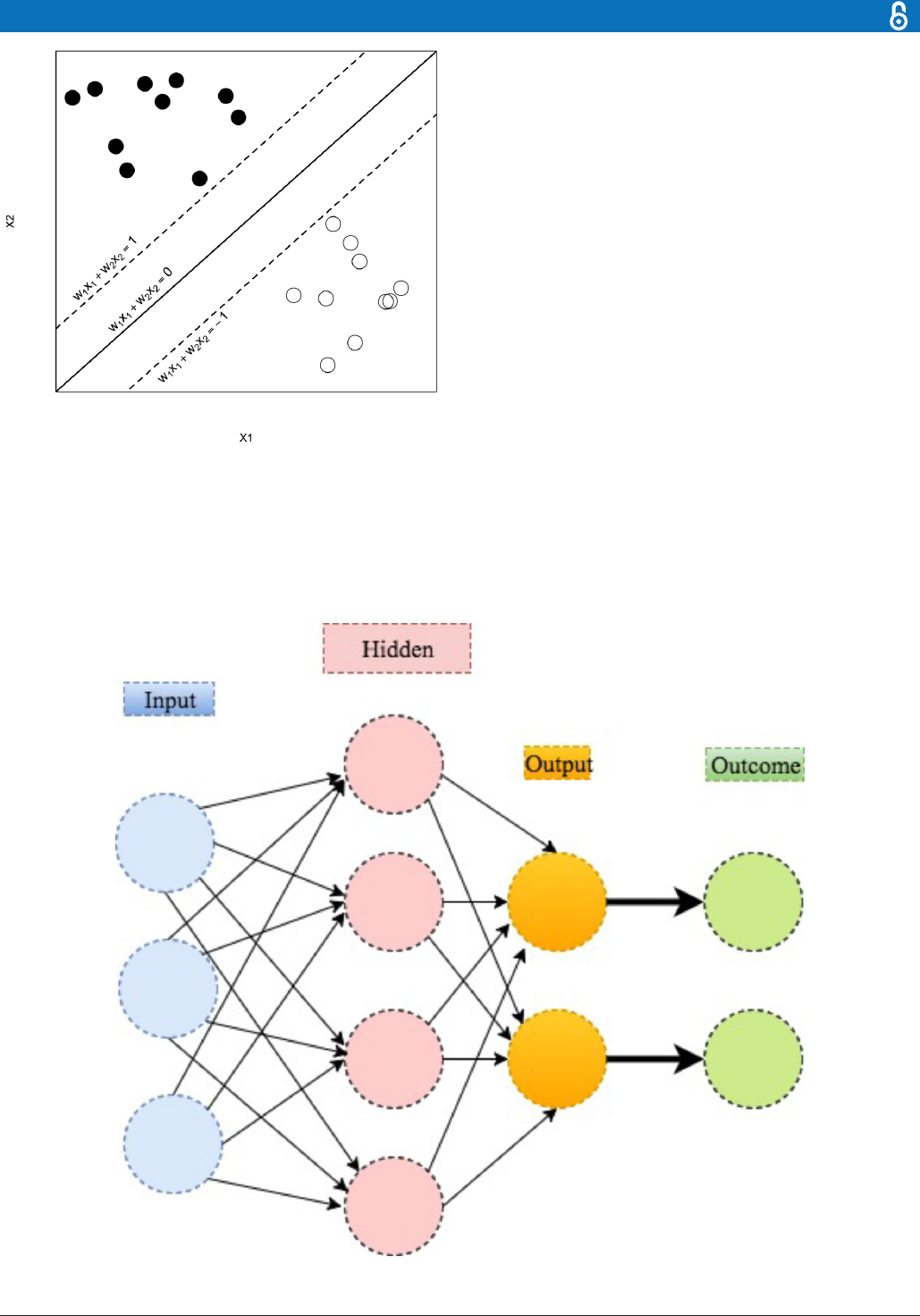
Support vector machine
SVM is mainly used for classifying the subjects into two
groups, where the outcome Y
i
is a classifier: Y
i
= −1 or
1 represents whether the ith patient is in group 1 or 2,
respectively. (The method can be extended for scenarios
with more than two groups.) The basic assumption is that
the subjects can be separated into two groups through a
decision boundary defined on the traits X
ij
, which can be
written as:
a
i
=
∑
p
j=1
w
j
X
ij
+ b
,
where w
j
is the weight putting on the jth trait to manifest
its relative importance on affecting the outcome among
the others. The decision rule then follows that if a
i
>0,
the ith patient is classified to group 1, that is, labelling Y
i
= −1; if a
i
<0, the patient is classified to group 2, that is,
labelling Y
i
=1. The class memberships are indeterminate
for the points with a
i
=0. See figure 7 for an illustration
with
p =2
,
b =0
, a
1
=1, and a
2
=−1.
The training goal is to find the optimal w
j
s so that
the resulting classifications agree with the outcomes as
much as possible, that is, with the smallest misclassifi-
cation error, the error of classifying a patient into the
wrong group. Intuitively, the best weights must allow (1)
the sign of a
i
to be the same as Y
i
so the classification is
correct; and (2) |a
i
| to be far away from 0 so the ambiguity
of the classification is minimised. These can be achieved
by selecting w
j
s that minimise a quadratic loss function.
29
Furthermore, assuming that the new patients come from
the same population, the resulting w
j
s can be applied to
classify these new patients based on their traits.
An important property of SVM is that the determination
of the model parameters is a convex optimisation problem
so the solution is always global optimum. Furthermore,
many existing convex optimisation tools are readily appli-
cable for the SVM implementation. As such, SVM has been
extensively used in medical research. For instance, Orrù et
al applied SVM to identify imaging biomarkers of neurolog-
ical and psychiatric disease.
30
Sweilam et al reviewed the use
of SVM in the diagnosis of cancer.
31
Khedher et al used the
Figure 5 The machine learning algorithms used in the medical literature. The data are generated through searching the
machine learning algorithms within healthcare on PubMed.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

235
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
combination of SVM and other statistical tools to achieve
early detection of Alzheimer’s disease.
32
Farina et al used
SVM to test the power of an offline man/machine interface
that controls upper-limb prostheses.
22
Neural network
One can think about neural network as an extension of
linear regression to capture complex non-linear relation-
ships between input variables and an outcome. In neural
Figure 6 The machine learning algorithms used for imaging (upper), genetic (middle) and electrophysiological (bottom) data.
The data are generated through searching the machine learning algorithms foreach data type on PubMed.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from
236
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
network, the associations between the outcome and the
input variables are depicted through multiple hidden
layer combinations of prespecified functionals. The goal
is to estimate the weights through input and outcome
data so that the average error between the outcome and
their predictions is minimised. We describe the method
in the following example.
Mirtskhulava et al used neural network in stroke diag-
nosis.
33
In their analysis, the input variables X
i1
, . . . , X
ip
are p=16 stroke-related symptoms, including paraesthesia
of the arm or leg, acute confusion, vision, problems with
mobility and so on. The outcome Y
i
is binary: Y
i
=1/0 indi-
cates the ith patient has/does not have stroke. The output
parameter of interest is the probability of stroke, a
i
, which
carries the form of
a
i
= h
{
∑
D
k=1
w
2l
f
k
(
∑
p
l=1
w
1l
X
il
+ w
10
)+w
20
}
.
In the above equation, the w
10
and w
20
≠0 guarantee the
above form to be valid even when all X
ij
, f
k
are 0; the w
1l
and
w
2l
s are the weights to characterise the relative impor-
tance of the corresponding multiplicands on affecting
the outcome; the f
k
s and
h
are prespecified functionals to
manifest how the weighted combinations influence the
disease risk as a whole. A stylised illustration is provided
in figure 8.
The training goal is to find the weights w
ij
, which mini-
mise the prediction error
∑
n
i=1
(
Y
i
− a
i
)
2
. The minimis-
ation can be performed through standard optimisation
algorithms, such as local quadratic approximation or
Figure 7 An illustration of the support vector machine.
Figure 8 An illustration of neural network.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

237
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
gradient descent optimisation, that are included in both
MATLAB and R. If the new data come from the same
population, the resulting w
ij
can be used to predict the
outcomes based on their specific traits.
29
Similar techniques have been used to diagnose cancer
by Khan et al, where the inputs are the PCs estimated from
6567 genes and the outcomes are the tumour catego-
ries.
34
Dheeba et al used neural network to predict breast
cancer, with the inputs being the texture information
from mammographic images and the outcomes being
tumour indicators.
35
Hirschauer et al used a more sophis-
ticated neural network model to diagnose Parkinson’s
disease based on the inputs of motor, non-motor symp-
toms and neuroimages.
36
Deep learning: a new era of ML
Deep learning is a modern extension of the classical
neural network technique. One can view deep learning
as a neural network with many layers (as in figure 9).
Rapid development of modern computing enables deep
learning to build up neural networks with a large number
of layers, which is infeasible for classical neural networks.
As such, deep learning can explore more complex
non-linear patterns in the data. Another reason for the
recent popularity of deep learning is due to the increase of
the volume and complexity of data.
37
Figure 10 shows that
the application of deep learning in the field of medical
research nearly doubled in 2016. In addition, figure 11
shows that a clear majority of deep learning is used in
imaging analysis, which makes sense given that images are
naturally complex and high volume.
Different from the classical neural network, deep
learning uses more hidden layers so that the algorithms
can handle complex data with various structures.
27
In the
medical applications, the commonly used deep learning
algorithms include convolution neural network (CNN),
recurrent neural network, deep belief network and deep
neural network. Figure 12 depicts their trends and rela-
tive popularities from 2013 to 2016. One can see that the
CNN is the most popular one in 2016.
The CNN is developed in viewing of the incompetence
of the classical ML algorithms when handling high dimen-
sional data, that is, data with a large number of traits. Tradi-
tionally, the ML algorithms are designed to analyse data
when the number of traits is small. However, the image
data are naturally high-dimensional because each image
normally contains thousands of pixels as traits. One solu-
tion is to perform dimension reduction: first preselect
a subset of pixels as features, and then perform the ML
algorithms on the resulting lower dimensional features.
However, heuristic feature selection procedures may lose
information in the images. Unsupervised learning tech-
niques such as PCA or clustering can be used for data-
driven dimension reduction.
The CNN was first proposed and advocated for the
high-dimensional image analysis by Lecun et al.
38
The
inputs for CNN are the properly normalised pixel values
on the images. The CNN then transfers the pixel values
in the image through weighting in the convolution layers
and sampling in the subsampling layers alternatively.
The final output is a recursive function of the weighted
Figure 9 An illustration of deep learning with two hidden layers.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

238
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
input values. The weights are trained to minimise the
average error between the outcomes and the predic-
tions. The implementation of CNN has been included in
popular software packages such as Caffe from Berkeley AI
Research,
39
CNTK from Microsoft
40
and TensorFlow from
Google.
41
Recently, the CNN has been successfully implemented
in the medical area to assist disease diagnosis. Long et al
used it to diagnose congenital cataract disease through
learning the ocular images.
24
The CNN yields over 90%
accuracy on diagnosis and treatment suggestion. Esteva
et al performed the CNN to identify skin cancer from
clinical images.
20
The proportions of correctly predicted
malignant lesions (ie, sensitivity) and benign lesions (ie,
specificity) are both over 90%, which indicates the supe-
rior performance of the CNN. Gulshan et al applied the
CNN to detect referable diabetic retinopathy through
the retinal fundus photographs.
25
The sensitivity and
specificity of the algorithm are both over 90%, which
demonstrates the effectiveness of using the technique
on the diagnosis of diabetes. It is worth mentioning that
in all these applications, the performance of the CNN is
Figure 10 Current trend for deep learning. The data are generated through searching the deep learning in healthcare and
disease category on PubMed.
Figure 11 The data sources for deep learning. The data are generated through searching deep learning in combination with
the diagnosis techniques on PubMed.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

239
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
competitive against experienced physicians in the accu-
racy for classifying both normal and disease cases.
Natural language processing
The image, EP and genetic data are machine-understand-
able so that the ML algorithms can be directly performed
after proper preprocessing or quality control processes.
However, large proportions of clinical information are in
the form of narrative text, such as physical examination,
clinical laboratory reports, operative notes and discharge
summaries, which are unstructured and incomprehen-
sible for the computer program. Under this context, NLP
targets at extracting useful information from the narra-
tive text to assist clinical decision making.
28
An NLP pipeline comprises two main components:
(1) text processing and (2) classification. Through text
processing, the NLP identifies a series of disease-relevant
keywords in the clinical notes based on the historical
databases.
42
Then a subset of the keywords are selected
through examining their effects on the classification of
the normal and abnormal cases. The validated keywords
then enter and enrich the structured data to support clin-
ical decision making.
The NLP pipelines have been developed to assist clin-
ical decision making on alerting treatment arrangements,
monitoring adverse effects and so on. For example,
Fiszman et al showed that introducing NLP for reading
the chest X-ray reports would assist the antibiotic assistant
system to alert physicians for the possible need for anti-in-
fective therapy.
43
Miller et al used NLP to automatically
monitor the laboratory-based adverse effects.
44
Further-
more, the NLP pipelines can help with disease diagnosis.
For instance, Castro et al identified 14 cerebral aneurysms
disease-associated variables through implementing NLP
on the clinical notes.
45
The resulting variables are success-
fully used for classifying the normal patients and the
patients with cerebral, with 95% and 86% accuracy rates
on the training and validation samples, respectively. Afzal
et al implemented the NLP to extract the peripheral arte-
rial disease-related keywords from narrative clinical notes.
The keywords are then used to classify the normal and the
patients with peripheral arterial disease, which achieves
over 90% accuracy.
42
AI APPLICATIONS IN STROKE
Stroke is a common and frequently occurring disease that
affects more than 500 million people worldwide. It is the
leading cause of death in China and the fifth in North
America. Stroke had cost about US$689 billion in medical
expenses across the world, causing heavy burden to coun-
tries and families.
46 47
Therefore, research on prevention
and treatment for stroke has great significance. In recent
years, AI techniques have been used in more and more
stroke-related studies. Below we summarise some of the
relevant AI techniques in the three main areas of stroke
care: early disease prediction and diagnosis, treatment,
as well as outcome prediction and prognosis evaluation.
Early detection and diagnosis
Stroke, for 85% of the time, is caused by thrombus in
the vessel called cerebral infarction. However, for lack of
judgement of early stroke symptom, only a few patients
could receive timely treatment. Villar et al developed a
movement-detecting device for early stroke prediction.
48
Two ML algorithms — genetic fuzzy finite state machine
Figure 12 The four main deep learning algorithm and their popularities. The data are generated through searching algorithm
names in healthcare and disease category on PubMed.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

240
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
and PCA — were implemented into the device for the
model building solution. The detection process included
a human activity recognition stage and a stroke-onset
detection stage. Once the movement of the patient is
significantly different from the normal pattern, an alert
of stroke would be activated and evaluated for treatment
as soon as possible. Similarly, Maninini et al proposed a
wearable device for collecting data about normal/patho-
logical gaits for stroke prediction.
49
The data would be
extracted and modelled by hidden Markov models and
SVM, and the algorithm could correctly classify 90.5% of
the subjects to the right group.
For diagnosis of stroke, neuroimaging techniques,
including MRI and CT, are important for disease evalu-
ation. Some studies have tried to apply ML methods to
neuroimaging data to assist with stroke diagnosis. Rehme
et al used SVM in resting-state functional MRI data, by
which endophenotypes of motor disability after stroke
were identified and classified.
50
SVM can correctly clas-
sify patients with stroke with 87.6% accuracy. Griffis
et al tried naïve Bayes classification to identify stroke
lesion in T1-weighted MRI.
51
The result is comparable
with human expert manual lesion delineation. Kamnitsas
et al tried three-dimensional CNN (3D CNN) for lesion
segmentation in multimodel brain MRI.
52
They also used
fully connected conditional random field model for final
postprocessing of the CNN’s soft segmentation maps.
Rondina et al analysed stroke anatomical MRI images
using Gaussian process regression, and found that the
patterns of voxels performed better than lesion load per
region as the predicting features.
53
ML methods have also been applied to analyse CT scans
from patients with stroke. Free-floating intraluminal
thrombus may be formed as lesion after stroke, which is
difficult to be distinguished with carotid plaque on the
CT imaging. Thornhill et al used three ML algorithms
to classify these two types by quantitative shape analysis,
including linear discriminant analysis, artificial neural
network and SVM.
54
The accuracy for each method varies
between 65.2% and 76.4%.
Treatment
ML has also been applied for predicting and analysing
the performance of stroke treatment. As a critical step
of emergency measure, the outcome of intravenous
thrombolysis (tPA) has strong relationship with the prog-
nosis and survival rate. Bentley et al used SVM to predict
whether patients with tPA treatment would develop symp-
tomatic intracranial haemorrhage by CT scan.
55
They
used whole-brain images as the input into the SVM, which
performed better than conventional radiology-based
methods. To improve the clinical decision-making process
of tPA treatment, Love et al proposed a stroke treatment
model by analysing practice guidelines, meta-analyses
and clinical trials using Bayesian belief network.
56
The
model consisted of 56 different variables and three deci-
sions for analysing the procedure of diagnosis, treatment
and outcome prediction. Ye et al used interaction trees
and subgroup analysis to explore appropriate tPA dosage
based on patient characteristics, taking into account both
the risk of bleeding and the treatment efficacy.
57
Outcome prediction and prognosis evaluation
Many factors can affect stroke prognosis and disease
mortality. Compared with conventional methods, ML
methods have advantages in improving prediction perfor-
mance. To better support clinical decision-making process,
Zhang et al proposed a model for predicting 3-month
treatment outcome by analysing physiological parameters
during 48 hours after stroke using logistic regression.
58
Asadi et al compiled a database of clinical information of
107 patients with acute anterior or posterior circulation
stroke who underwent intra-arterial therapy.
59
The authors
analysed the data via artificial neural network and SVM, and
obtained prediction accuracy above 70%. They also used
ML techniques to identify factors influencing outcome
in brain arteriovenous malformation treated with endo-
vascular embolisation.
60
While standard regression anal-
ysis model could only achieve a 43% accuracy rate, their
methods worked much better with 97.5% accuracy.
Birkner et al used an optimal algorithm to predict 30-day
mortality and obtained more accurate prediction than
existing methods.
61
Similarly, King et al used SVM to predict
stroke mortality at discharge.
62
In addition, they proposed
the use of the synthetic minority oversampling technique
to reduce the stroke outcome prediction bias caused by
between-class imbalance among multiple data sets.
Brain images have been analysed to predict the outcome
of stroke treatment. Chen et al analysed CT scan data via
ML for evaluating the cerebral oedema following hemi-
spheric infarction.
63
They built random forest to automat-
ically identify cerebrospinal fluid and analyse the shifts
on CT scan, which is more efficient and accurate than
conventional methods. Siegel et al extracted functional
connectivity from MRI and functional MRI data, and used
ridge regression and multitask learning for cognitive defi-
ciency prediction after stroke.
64
Hope et al studied the
relationship between lesions extracted from MRI images
and the treatment outcome via Gaussian process regres-
sion model.
65
They used the model to predict the severity
of cognitive impairments after stroke and the course of
recovery over time.
CONCLUSION AND DISCUSSION
We reviewed the motivation of using AI in healthcare,
presented the various healthcare data that AI has analysed
and surveyed the major disease types that AI has been
deployed. We then discussed in details the two major cate-
gories of AI devices: ML and NLP. For ML, we focused
on the two most popular classical techniques: SVM and
neural network, as well as the modern deep learning tech-
nique. We then surveyed the three major categories of AI
applications in stroke care.
A successful AI system must possess the ML component
for handling structured data (images, EP data, genetic
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

241
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
data) and the NLP component for mining unstructured
texts. The sophisticated algorithms then need to be
trained through healthcare data before the system can
assist physicians with disease diagnosis and treatment
suggestions.
The IBM Watson system is a pioneer in this field. The
system includes both ML and NLP modules, and has
made promising progress in oncology. For example, in
a cancer research, 99% of the treatment recommenda-
tions from Watson are coherent with the physician deci-
sions.
66
Furthermore, Watson collaborated with Quest
Diagnostics to offer the AI Genetic Diagnostic Analysis.
66
In addition, the system started to make impact on actual
clinical practices. For example, through analysing genetic
data, Watson successfully identified the rare secondary
leukaemia caused by myelodysplastic syndromes in
Japan.
67
The cloud-based CC-Cruiser in
24
can be one prototype
to connect an AI system with the front-end data input
and the back-end clinical actions. More specifically, when
patients come, with their permission, their demographic
information and clinical data (images, EP results, genetic
results, blood pressure, medical notes and so on) are
collected into the AI system. The AI system then uses the
patients’ data to come up with clinical suggestions. These
suggestions are sent to physicians to assist with their clin-
ical decision making. Feedback about the suggestions
(correct or wrong) will also be collected and fed back into
the AI system so that it can keep improving accuracy.
Stroke is a chronic disease with acute events. Stroke
management is a rather complicated process with a series
of clinical decision points. Traditionally clinical research
solely focused on a single or very limited clinical questions,
while ignoring the continuous nature of stroke manage-
ment. Taking the advantage of large amount of data with
rich information, AI is expected to help with studying
much more complicated yet much closer to real-life clin-
ical questions, which then leads to better decision making
in stroke management. Recently, researchers have started
endeavours along this direction and obtained promising
initial results.
57
Although the AI technologies are attracting substantial
attentions in medical research, the real-life implementa-
tion is still facing obstacles. The first hurdle comes from
the regulations. Current regulations lack of standards
to assess the safety and efficacy of AI systems. To over-
come the difficulty, the US FDA made the first attempt to
provide guidance for assessing AI systems.
68
The first guid-
ance classifies AI systems to be the ‘general wellness prod-
ucts’, which are loosely regulated as long as the devices
intend for only general wellness and present low risk to
users. The second guidance justifies the use of real-world
evidence to access the performance of AI systems. Lastly,
the guidance clarifies the rules for the adaptive design in
clinical trials, which would be widely used in assessing the
operating characteristics of AI systems. Not long after the
disclosure of these guidances, Arterys’ medical imaging
platform became the first FDA-approved deep learning
clinical platform that can help cardiologists to diagnose
cardiac diseases.
23
The second hurdle is data exchange. In order to work
well, AI systems need to be trained (continuously) by
data from clinical studies. However, once an AI system
gets deployed after initial training with historical data,
continuation of the data supply becomes a crucial issue
for further development and improvement of the system.
Current healthcare environment does not provide incen-
tives for sharing data on the system. Nevertheless, a health-
care revolution is under way to stimulate data sharing in
the USA.
69
The reform starts with changing the health
service payment scheme. Many payers, mostly insurance
companies, have shifted from rewarding the physicians by
shifting the treatment volume to the treatment outcome.
Furthermore, the payers also reimburse for a medica-
tion or a treatment procedure by its efficiency. Under
this new environment, all the parties in the healthcare
system, the physicians, the pharmaceutical companies
and the patients, have greater incentives to compile and
exchange information. Similar approaches are being
explored in China.
Correction notice This paper has been corrected since it was published Online
First. Owing to a scripting error, some of the publisher names in the references
were replaced with 'BMJ Publishing Group'. This only affected the full text version,
not the PDF. We have since corrected these errors and the correct publishers have
been inserted into the references. Figures 6-9 have also been corrected.
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.
Data sharing statement No additional data are available.
Open Access This is an Open Access article distributed in accordance with the
Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which
permits others to distribute, remix, adapt, build upon this work non-commercially,
and license their derivative works on different terms, provided the original work is
properly cited and the use is non-commercial. See: http:// creativecommons. org/
licenses/ by- nc/ 4. 0/
© Article author(s) (or their employer(s) unless otherwise stated in the text of the
article) 2017. All rights reserved. No commercial use is permitted unless otherwise
expressly granted.
RefeRences
1. Murdoch TB, Detsky AS. The inevitable application of big data to
health care. JAMA 2013;309:1351–2.
2. Kolker E, Özdemir V, Kolker E. How Healthcare can refocus on its
Super-Customers (Patients, n =1) and Customers (Doctors and
Nurses) by Leveraging Lessons from Amazon, Uber, and Watson.
OMICS 2016;20:329–33.
3. Dilsizian SE, Siegel EL. Articial intelligence in medicine and cardiac
imaging: harnessing big data and advanced computing to provide
personalized medical diagnosis and treatment. Curr Cardiol Rep
2014;16:441.
4. Patel VL, Shortliffe EH, Stefanelli M, et al. The coming of age of
articial intelligence in medicine. Artif Intell Med 2009;46:5–17.
5. Jha S, Topol EJ. Adapting to Articial Intelligence: radiologists and
pathologists as information specialists. JAMA 2016;316:2353–4.
6. Pearson T. How to replicate Watson hardware and systems design
for your own use in your basement. 2011 https://www. ibm. com/
developerworks/ community/ blogs/ InsideSystemStorage/ entry/ ibm_
watson_ how_ to_ build_ your_ own_ watson_ jr_ in_ your_ basement7?
lang= en (accessed 1 Jun 2017).
7. Weingart SN, Wilson RM, Gibberd RW, et al. Epidemiology of medical
error. BMJ 2000;320:774–7.
8. Graber ML, Franklin N, Gordon R. Diagnostic error in internal
medicine. Arch Intern Med 2005;165:1493–9.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

242
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
9. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the
intensive care unit: a systematic review of autopsy studies. BMJ Qual
Saf 2012;21:894–902.
10. Lee CS, Nagy PG, Weaver SJ, et al. Cognitive and system factors
contributing to diagnostic errors in radiology. AJR Am J Roentgenol
2013;201:611–7.
11. Neill DB. Using artificial intelligence to improve hospital inpatient
care. IEEE Intell Syst 2013;28:92–5.
12. Administration UFaD. Guidance for industry: electronic source data
in clinical investigations. 2013 https://www. fda. gov/ downloads/
drugs/ guidances/ ucm328691. pdf (accessed 1 Jun 2017).
13. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than
pictures, they are data. Radiology 2016;278:563–77.
14. Li CY, Liang GY, Yao WZ, et al. Integrated analysis of long non-
coding RNA competing interactions reveals the potential role in
progression of human gastric Cancer. Int J Oncol 2016;48:1965–76.
15. Shin H, Kim KH, Song C, et al. Electrodiagnosis support system for
localizing neural injury in an upper limb. J Am Med Inform Assoc
2010;17:345–7.
16. Karakülah G, Dicle O, Koşaner O, et al. Computer based extraction of
phenoptypic features of human congenital anomalies from the digital
literature with natural language processing techniques. Stud Health
Technol Inform 2014;205:570–4.
17. Darcy AM, Louie AK, Roberts LW. Machine Learning and the
Profession of Medicine. JAMA 2016;315:551–2.
18. Murff HJ, FitzHenry F, Matheny ME, et al. Automated identication
of postoperative complications within an electronic medical record
using natural language processing. JAMA 2011;306:848–55.
19. Somashekhar SP, Kumarc R, Rauthan A, et al. Abstract S6-07:
double blinded validation study to assess performance of IBM
articial intelligence platform, Watson for oncology in comparison
with manipal multidisciplinary tumour board ? rst study of 638
breast Cancer cases. Cancer Res 2017;77(4 Suppl):S6-07.
20. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level
classication of skin Cancer with deep neural networks. Nature
2017;542:115–8.
21. Bouton CE, Shaikhouni A, Annetta NV, et al. Restoring cortical
control of functional movement in a human with quadriplegia. Nature
2016;533:247–50.
22. Farina D, Vujaklija I, Sartori M, et al. Man/machine interface based on
the discharge timings of spinal motor neurons after targeted muscle
reinnervation. Nat Biomed Eng 2017;1:0025.
23. Marr B. First FDA approval for clinical Cloud-Based Deep Learning in
Healthcare. 2017. https://www. forbes. com/ sites/ bernardmarr/ 2017/
01/ 20/ rst- fda- approval- for- clinical- cloud- based- deep- learning- in-
healthcare/# 7a0ed8dc161c (accessed 1 Jun 2017).
24. Long E, Lin H, Liu Z, et al. An articial intelligence platform for the
multihospital collaborative management of congenital cataracts,
2017.
25. Gulshan V, Peng L, Coram M, et al. Development and Validation of
a Deep Learning Algorithm for detection of Diabetic Retinopathy in
retinal fundus photographs. JAMA 2016;316:2402–10.
26. James G, Witten D, Hastie T, et al. An introduction to Statistical
Learning with applications in R. First Edition: Springer, 2013.
27. Goodfellow I, Bengio Y, Courville A. Deep Learning. First Edition: The
MIT Press, 2016.
28. Kantor P. Foundations of statistical natural language processing: MIT
Press, 1999:91–2.
29. Bishop CM, ed. Pattern recognition and machine Learning
(Information Science and Statistics, 2007.
30. Orrù G, Pettersson-Yeo W, Marquand AF, et al. Using support
Vector Machine to identify imaging biomarkers of neurological
and psychiatric disease: a critical review. Neurosci Biobehav Rev
2012;36:1140–52.
31. Sweilam NH, Tharwat AA, Abdel Moniem NK, Moniem NKA. Support
vector machine for diagnosis Cancer disease: a comparative study.
Egyptian Informatics Journal 2010;11:81–92.
32. Khedher L, Ram?rez J, G?rriz JM, et al. Early diagnosis of
Alzheimer?s disease based on partial least squares, principal
component analysis and support vector machine using segmented
MRI images. Neurocomputing 2015;151:139–50.
33. In: Mirtskhulava L, Wong J, Al-Majeed S, Pearce G, et al; eds.
Articial Neural Network Model in Stroke diagnosis. modelling
and simulation (UKSim), 2015 17th UKSim-AMSS International
Conference on: IEEE, 2015.
34. Khan J, Wei JS, Ringnér M, et al. Classication and diagnostic
prediction of cancers using gene expression proling and articial
neural networks. Nat Med 2001;7:673–9.
35. Dheeba J, Albert Singh N, Tamil Selvi S. Computer-aided detection
of breast Cancer on mammograms: a swarm intelligence optimized
wavelet neural network approach. J Biomed Inform 2014;49:45–52.
36. Hirschauer TJ, Adeli H, Buford JA. Computer-Aided diagnosis of
Parkinson's Disease Using Enhanced Probabilistic Neural Network. J
Med Syst 2015;39:179.
37. Ravi D, Wong C, Deligianni F, et al. Deep Learning for Health
Informatics. IEEE J Biomed Health Inform 2017;21:4–21.
38. Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied
to document recognition. Proc IEEE Inst Electr Electron Eng
1998;86:2278–324.
39. Research BA. Caffe. 2017. http:// caffe. berkeleyvision. org/ (accessed
1 Jun 2017).
40. Seide F, Agarwal A, eds. CNTK: microsoft's Open-Source Deep-
Learning Toolkit. ACM SIGKDD International Conference on
Knowledge Discovery and Data Mining, 2016.
41. Abadi M, Agarwal A, Barham P, et al; TensorFlow: large-scale
Machine Learning on heterogeneous distributed Systems, 2016.
42. Afzal N, Sohn S, Abram S, et al. Mining peripheral arterial disease
cases from narrative clinical notes using natural language
processing. J Vasc Surg 2017;65:1753–61.
43. Fiszman M, Chapman WW, Aronsky D, et al. Automatic detection
of acute bacterial pneumonia from chest X-ray reports. J Am Med
Inform Assoc 2000;7:593–604.
44. Miller TP, Li Y, Getz KD, et al. Using electronic medical record data to
report laboratory adverse events. Br J Haematol 2017;177:283–6.
45. Castro VM, Dligach D, Finan S, et al. Large-scale identication of
patients with cerebral aneurysms using natural language processing.
Neurology 2017;88:164–8.
46. Saenger AK, Christenson RH. Stroke biomarkers: progress and
challenges for diagnosis, prognosis, differentiation, and treatment.
Clin Chem 2010;56:21–33.
47. Heeley E, Anderson CS, Huang Y, et al. Role of health insurance in
averting economic hardship in families after acute stroke in China.
Stroke 2009;40:2149–56.
48. Villar JR, González S, Sedano J, et al. Improving human activity
recognition and its application in early stroke diagnosis. Int J Neural
Syst 2015;25:1450036.
49. Mannini A, Trojaniello D, Cereatti A, et al. A machine Learning
Framework for Gait classication using inertial sensors: application
to Elderly, Post-Stroke and Huntington's Disease Patients. Sensors
2016;16:134.
50. Rehme AK, Volz LJ, Feis DL, et al. Identifying neuroimaging markers
of Motor Disability in acute stroke by machine Learning Techniques.
Cereb Cortex 2015;25:3046–56.
51. Grifs JC, Allendorfer JB, Szaarski JP. Voxel-based gaussian naïve
Bayes classication of ischemic stroke lesions in individual T1-
weighted MRI scans. J Neurosci Methods 2016;257:97–108.
52. Kamnitsas K, Ledig C, Newcombe VF, et al. Efcient multi-scale
3D CNN with fully connected CRF for accurate brain lesion
segmentation. Med Image Anal 2017;36:61–78.
53. Rondina JM, Filippone M, Girolami M, et al. Decoding post-stroke
motor function from structural brain imaging. Neuroimage Clin
2016;12:372–80.
54. Thornhill RE, Lum C, Jaberi A, et al. Can shape analysis differentiate
free-oating internal carotid artery Thrombus from atherosclerotic
plaque in patients evaluated with CTA?for stroke or transient
ischemic attack? Acad Radiol 2014;21:345–54.
55. Bentley P, Ganesalingam J, Carlton Jones AL, et al. Prediction of
stroke thrombolysis outcome using CT brain machine learning.
Neuroimage Clin 2014;4:635–40.
56. Love A, Arnold CW, El-Saden S, et al. Unifying acute stroke
treatment guidelines for a bayesian belief network. Stud Health
Technol Inform 2013;192:1012.
57. Ye H, Shen H, Dong Y, et al. Using Evidence-Based medicine through
Advanced Data Analytics to work toward a National Standard for
Hospital-based acute ischemic Stroke treatment. Mainland China,
2017.
58. Zhang Q, Xie Y, Ye P, et al. Acute ischaemic stroke prediction from
physiological time series patterns. Australas Med J 2013;6:280–6.
59. Asadi H, Dowling R, Yan B, et al. Machine learning for outcome
prediction of acute ischemic stroke post intra-arterial therapy. PLoS
One 2014;9:e88225.
60. Asadi H, Kok HK, Looby S, et al. Outcomes and complications after
endovascular treatment of Brain Arteriovenous Malformations: a
Prognostication Attempt using articial Intelligence. World Neurosurg
2016;96:562–9.
61. Birkner MD, Kalantri S, Solao V, et al. Creating diagnostic scores
using data-adaptive regression: an application to prediction of 30-
day mortality among stroke victims in a rural hospital in India. Ther
Clin Risk Manag 2007;3:475–84.
62. Ho KC, Speier W, El-Saden S, et al. Predicting discharge mortality
after acute ischemic stroke using balanced data. AMIA Annu Symp
Proc 2014;2014:1787–96.
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from

243
JiangF, etal. Stroke and Vascular Neurology 2017;2:e000101. doi:10.1136/svn-2017-000101
Open Access
63. Chen Y, Dhar R, Heitsch L, et al. Automated quantication of cerebral
edema following hemispheric infarction: application of a machine-
learning algorithm to evaluate CSF shifts on serial head CTs.
Neuroimage Clin 2016;12:673–80.
64. Siegel JS, Ramsey LE, Snyder AZ, et al. Disruptions of network
connectivity predict impairment in multiple behavioral domains after
stroke. Proc Natl Acad Sci USA 2016;113:E4367–E4376.
65. Hope TM, Seghier ML, Leff AP, et al. Predicting outcome and
recovery after stroke with lesions extracted from MRI images.
Neuroimage Clin 2013;2:424–33.
66. LOHR S. IBM is counting on its bet on Watson, and Paying Big
Money for It. 2016 https://www. nytimes. com/ 2016/ 10/ 17/ technology/
ibm- is- counting- on- its- bet- on- watson- and- paying- big- money- for- it.
html (accessed 1 Jun 2017).
67. Otake T. IBM Big Data used for rapid diagnosis of rare leukemia
case in Japan. 2016 http://www. japantimes. co. jp/ news/ 2016/ 08/ 11/
national/ science- health/ ibm- big- data- used- for- rapid- diagnosis- of-
rare- leukemia- case- in- japan (accessed 1 Jun 2017).
68. Graham J. Articial Intelligence, Machine Learning, and the FDA.
2016 https://www. forbes. com/ sites/ theapothecary/ 2016/ 08/ 19/
articial- intelligence- machine- learning- and- the- fda/# 4aca26121aa1
(accessed 1 Jun 2017).
69. Kayyali B, Knott D, Kuiken SV. The big-data revolution in US health
care: accelerating value and innovation. 2013 http://www. mckinsey.
com/ industries/ healthcare- systems- and- services/ our- insights/ the-
big- data- revolution- in- us- health- care (accessed 1 Jun 2017).
on October 7, 2024 by guest. Protected by copyright.http://svn.bmj.com/Stroke Vasc Neurol: first published as 10.1136/svn-2017-000101 on 21 June 2017. Downloaded from
