
®
Recognize. Rule-Out. Refer.
Biothreat Agent Bench Cards
for the Sentinel Laboratory
®
For questions, contact your designated LRN Reference Level Laboratory:
(LRN Reference Level Laboratory Name)
(Phone Number)

2
A
PHL thanks the Sentinel Laboratory Partnerships and Outreach Subcommittee, the Public
Health Preparedness and Response Committee, the American Society for Microbiology and
the US Centers for Disease Control and Prevention for contributing their time and expertise to
provide substantial guidance on the development of these bench cards.
Special thanks to the Florida Department of Health, Massachusetts State Public Health
Laboratory, Michigan Department of Health and Human Services, Minnesota Department of
Health, Oregon State Public Health Laboratory, San Antonio Metro Health District, Wadsworth
Center at the New York State Department for Health and Wisconsin State Laboratory of
Hygiene for providing subject matter expertise, content review and photos.
This project was 100% financed by federal funds. The total amount of funding received for the
Public Health Preparedness and Response program is $1,768,631.
This project was supported by Cooperative Agreement # NU60OE000103 from CDC. Its
contents are solely the responsibility of the author and do not necessarily represent the
official views of the CDC.

3
INSTITUTION / LRN REF LABORATORY:
Address:
Phone Number:
Website:
EMERGENCY NUMBERS
Laboratory (business hours):
Laboratory (after hours):
Biothreat Coordinator:
Epidemiology Dept. (business hours):
Epidemiology Dept. (after hours):
Duty Ofcer/Other On-Call:
STATE / LOCAL PUBLIC HEALTH LABORATORY DEPARTMENTS
Microbiology:
Virology:
Serology:
Specimen Receiving/Packaging:
State-Specific Information

4
Safety
Safety Precautions ...................................................................... 5
Preventing Aerosolization ........................................................... 6
Responding to a Biothreat Agent
Laboratory Response Network for Biological Threats............... 7
Responsibilities of the Sentinel Laboratory .............................. 8
Sentinel Laboratory Checklists .................................................. 9
Biological Risk Assessments ....................................................10
Using BSL-3 Practices ...............................................................11
Biothreat Agent Response Algorithm ....................................... 12
Biothreat agent Identification
Gram Negative Bacilli/Cocobacilli Rule-Out Algorithm ...........13
Anthrax — Bacillus anthracis
Handling Instructions ................................................................14
Characterization ........................................................................15
Rule-Out Algorithm ....................................................................16
Anthrax — Bacillus cereus biovar anthracis
Characterization ........................................................................ 17
Recommendations .................................................................... 18
Brucellosis — Brucella spp.
Handling Instructions ................................................................19
Characterization ........................................................................20
Rule-Out Algorithm ....................................................................21
Glanders — Burkholderia mallei
Handling Instructions ................................................................22
Characterization ........................................................................23
Rule-Out Algorithm .................................................................... 24
Melioidosis — Burkholderia pseudomallei
Handling Instructions ................................................................22
Characterization ........................................................................25
Rule-Out Algorithm ....................................................................26
Tularemia — Francisella tularensis
Handling Instructions ................................................................ 27
Characterization ........................................................................28
Rule-Out Algorithm ....................................................................29
Plague — Yersinia pestis
Handling Instructions ................................................................30
Characterization ........................................................................ 31
Rule-Out Algorithm ....................................................................32
Appendix
Acronyms ...................................................................................33
Terms and Denitions ............................................................... 34
Identication Tests .................................................................... 37
Resources .................................................................................. 39
TABLE OF CONTENTS
Refer to the ASM Sentinel Laboratory Guidelines and consult with your LRN Reference Laboratory
for other suspect biothreat organisms not routinely seen in the Sentinel Laboratory, such as
Clostridium botulinum, novel inuenza, Smallpox, Staphylococcus aureus enterotoxin B (SEB), Coxiella burnetii, etc.

5
Identification Systems
Use May Result in Exposure or Misidentification of Biothreat Agents
Using automated or manual identication systems (e.g., MALDI-TOF, Vitek, API 20 NE, Bactec) may result in
exposure to dangerous pathogens, and could result in erroneous identication (e.g., Bacillus anthracis misidentied
as B. cereus; Yersinia pestis misidentied as Y. pseudotuberculosis, etc.).
Filter Extract to Reduce Risk of Contamination or Exposure
If using automated identication systems for bacterial identication and the manufacturer provided an alternate
tube extraction method (most common with MALDI-TOF), it is recommended that the resulting extract be ltered
using a 0.2 μm (or less) lter. This additional step will reduce the risk of laboratory contamination with viable
bacteria and spores.
Handling a Suspected Biothreat Agent
Use a Biological Safety Cabinet & BSL-3 Practices
As soon as a biothreat agent is suspected, perform all further work in a certied Class II BSC using BSL-3 practices
and appropriate BSL-3 PPE.
Contact your LRN Reference Level Lab
If the agent cannot be ruled-out with tests listed within these bench cards, do not attempt further identication
using commercial automated or kit identication systems. Contact your LRN Reference Level Laboratory to refer the
isolate.
Safety Precautions
SAFETY

6
Aerosolization
Aerosolization can occur during any procedure
which imparts energy into a microbial suspension,
producing aerosols or droplets which may contain
infectious organisms. Aerosols are very small
particles that may remain suspended in the air and
can be inhaled and retained in the lungs. Droplets
are larger particles which can settle onto surfaces
and gloves due to gravity. Droplets may also come
into contact with the mucous membranes of the
person performing the procedure.
Safety Precautions
Laboratory exposures can be decreased by working
in a BSC using BSL-3 practices and appropriate
BSL-3 PPE when a biothreat agent is suspected.
Identied aerosol-generating procedure risks
should be mitigated.
Examples of Aerosol Producing Procedures
• Opening culture plate, snifng plates
(Examining colony morphology/growth)
• Heat xing a slide
• Dispensing pipette tips
• Centrifuge setup/run/unloading
• Vortexing
• Spills or splashes of liquid media
• Subculturing positive blood culture bottles
• Inoculation of media (plate or tube)
• Preparing samples for automated ID systems
• Open ames, sterilizing loops
• Sonicating
• Pipetting
• Catalase test
• Using automated and manual identication
systems (e.g., MALDI-TOF, Vitek, API 20 NE,
Bactec)
Your facility may identify additional aerosol generating procedures based on the laboratory's risk assessments.
Preventing Aerosolization
SAFETY

7
The LRN-B was founded in 1999 by CDC, FBI and APHL
to coordinate laboratory response to biological, chemical,
radiological threats and other high priority public health
emergencies, including emerging infectious diseases.
National Laboratories
National labs, including the CDC, US Army Medical Research
Institute of Infectious Diseases (USAMRIID), and the Naval
Medical Research Center (NMRC), are responsible for
specialized strain characterization, bioforensics, biothreat agent
activity and handling of highly infectious biological agents.
Reference Laboratories
Reference labs, including state and local public health, military,
veterinary, agriculture, food and water testing laboratories, are
responsible for investigation and conrmatory testing. Facilities
located in Australia, Canada, the United Kingdom, Mexico and
South Korea serve as international reference laboratories.
Sentinel Laboratories
Sentinel labs, comprised of hospital-based and commercial
laboratories, are responsible for the early detection and the
rule-out or referral of potential biothreat agents.
Laboratory Response Network for Biological Threats
RESPONDING TO A BIOTHREAT AGENT

8
A Sentinel Laboratory:
1. Is familiar with reportable disease guidelines in its jurisdiction, and
has policies and procedures in place to refer clinical and diagnostic
specimens or isolates suspected to contain agents of public health
signicance to the appropriate local or state public health laboratory.
2. Ensures sufcient personnel have met the applicable federal
regulations for packaging and shipping of Category A and B
infectious substances.
3. Has policies and procedures for the collection and referral of
suspect biothreat agents or other emerging threat specimens and/
or isolates to the appropriate LRN Reference Laboratory consistent
with the ASM Sentinel Level Clinical Laboratory Protocols and
Guidelines for Suspected Agents of Bioterrorism and Emerging
Infectious Diseases.
4. If a clinical core laboratory, provides their satellite facilities with
written directions and training as needed for appropriate specimen
collection and handling. Core laboratories should also provide
satellite facilities with procedures for the recognition of the agents
of bioterrorism and assure training at a level commensurate with the
complexity of services offered at that facility.
5. Maintains the capability to perform the testing outlined in the ASM
Sentinel Clinical Laboratory Protocols and must demonstrate annual
competency by participation in prociency testing or exercises, such
as APHL, CDC and the College of American Pathologists Laboratory
Preparedness Exercise (CAP LPX), state-developed prociency/
challenge sets, or other equivalent assessment.
6. Based on its risk assessment, has and utilizes a currently certied
Class II or higher BSC when there is a risk of aerosol production or
when working with a biological threat agent or other emerging threat
organism is suspected.
7. Complies with the practices as outlined in the current edition of the
Biosafety in Microbiological and Biomedical Laboratories guidelines
and those detailed in the Guidelines for Safe Work Practices in
Human and Animal Medical Diagnostic Laboratories.
8. Has a biosafety and biosecurity risk assessment policy and ensures
that such risk assessments are routinely performed as part of their
quality management program.
9. Complies with applicable US Occupational Safety and Health
Administration regulations for bloodborne pathogens and has a
respiratory protection program.
10. Complies with the applicable rules and regulations of the Federal
Select Agent Program.
11. Has policies and procedures for secure storage of any remaining
suspect biothreat or other emerging threat agent material retained
within its facilities until it is transferred or destroyed.
12. Has policies and procedures for nal decontamination/destruction
of any remaining suspect biothreat or other emerging threat agent
material within the required time-frame (e.g., primary specimens or
subcultures retained within its facilities).
Responsibilities of the Sentinel Laboratory
RESPONDING TO A BIOTHREAT AGENT

9
Laboratory Preparedness
Plans
□
Institutional Emergency or Incident
Response Plan
□
Specic Bioterrorism Response Plan
□
Institutional Risk Assessment Plan
Training
□
Packaging and Shipping of Infectious
Substances
□
Rule-Out of Select/Biothreat Agents
□
Select Agent Regulations
□
Communications and Messaging
Proficiency Testing
□
Prociency test/exercise
(e.g., CAP LPX)
□
Maintain supplies for rule-out testing
Updates
□
Review ASM’s website for updated
Sentinel Level Clinical Laboratory
Protocols
□
APHL trainings
If you have a:
Suspect BT Agent
□
Follow rule-out procedures and
conduct work in a BSC
□
Initiate/maintain communication
with departmental/hospital
leadership and infection control
□
Contact BT personnel at designated
LRN Reference Level Laboratory
□
Ship isolate to designated LRN
Reference Level Laboratory
□
Document courier transfer
(e.g., institutional or commercial
courier tracking number)
□
Secure all potential biothreat
agent(s) and residual samples
□
Document personnel with access
to potential biothreat agent(s)
(biosecurity)
□
Document personnel who have
worked with suspect biothreat agent
and those present in laboratory if
exposure occurred (biosafety)
Confirmed BT Agent
□
Follow directions from designated
LRN Reference Level Laboratory
for the destruction or transfer of all
isolates/specimens
□
Perform risk assessment review
□
Document identication of biothreat
agent(s) with APHIS/CDC Form 4
□
Document disposition of biothreat
agent(s) with APHIS/CDC forms:
• Form 2 to transfer
• Form 4 for destruction
Exposure to a BT Agent:
□
Document any laboratory exposures
with APHIS/CDC Form 3
□
Work with designated LRN
Reference Level Laboratory or
health department for post-exposure
prophylaxis
Sentinel Laboratory Checklists
RESPONDING TO A BIOTHREAT AGENT

10
Biological Risk Assessment Goals
• Identify hazards associated with handling infectious agents in the
laboratory.
• Identify and implement controls in order to minimize the risk of
exposure to workers and the environment.
• In the clinical lab, focus is primarily on the prevention of laboratory
acquired infections from:
• Spills/splashes to mucous membranes
• Inhalation of aerosols
• Percutaneous inoculation from cuts, needle sticks,
non-intact skin
• Ingestion (e.g., contamination from surfaces, fomites to hands, etc.)
Conducting a Biological Risk Assessment
Risk assessments must be performed regularly based on procedure or
agent, and when there are changes in agents, procedures, equipment
or staff. Risks identied by the assessment should be prioritized and a
mitigation plan should be established based on that prioritization.
Risk assessments require management involvement and support,
knowledge of the hazards and understanding of the work, the environment
and the staff. Ideally, they consist of a multidisciplinary team, depending on
the work.
Consult with your LRN Reference Lab for guidance, and refer to APHL's Risk
Assessment Best Practices for more information.
Biological Risk Assessments
RESPONDING TO A BIOTHREAT AGENT

11
BSL-3 Practices
• Restrict access to the laboratory.
• Wear additional PPE (solid-front gown, gloves and face/eye protection as a minimum) and respiratory protection
(previously t-tested for use).
• Laboratory personnel must demonstrate prociency prior to handling pathogenic and potentially lethal agents, and
must be supervised by scientists experienced and competent in handling the specic infectious agents present in the
laboratory and associated procedures.
• Do not manipulate organisms or work in open vessels on the bench. All work must take place in a certied Class II or
higher BSC, or other containment equipment. Tape plates shut.
• Evaluate all potential exposures immediately.
• Decontaminate all cultures, stocks and other potentially infectious materials prior to disposal by using an approved
decontamination method, such as autoclaving or chemical disinfection. Decontamination would preferably take place
within the laboratory.
When to Use BSL-3 Practices in a BSL-2 Laboratory
• When working with agents that can be transmitted via inhalation and are normally handled at BSL-3, but a BSL-3
laboratory is not readily available.
• When the laboratory director determines that BSL-3 practices are needed based on a risk assessment.
• When specic high-risk pathogenic organisms are suspected, such as Brucella spp., Coccidioides spp., Blastomyces
dermatitidis, Francisella tularensis, Histoplasma capsulatum, Mycobacterium tuberculosis, MERS, SARS, highly
pathogenic inuenza, Tier 1 Select Agents, etc.
Using BSL-3 Practices
RESPONDING TO A BIOTHREAT AGENT

12
Perform rule-out testing.
Able to rule-out biothreat agent?
Able to rule-in/conrm
biothreat agent?
Proceed with
routine ID
Notify LRN Reference Laboratory by phone and then ship them
the suspect select agent, using appropriate procedures.
Immediately secure any remaining specimens, isolates and
derivatives until Reference Laboratory's testing is completed.
Notify the Sentinel Laboratory and inquire about
potential biothreat agent exposures there.
Initiate
APHIS/CDC
Form 3
Initiate APHIS/CDC Form 4;
complete sections A & B,
send to Sentinel Laboratory.
If required, immediately
notify CDC.
Complete sections C & D of APHIS/CDC Form 4
and return to LRN Reference Laboratory
(which will send it to CDC Select Agent Program).
If requested, transfer all
specimens, isolates and
derivatives to the Reference
Laboratory, which will
initiate APHIS/CDC Form 2.
Complete Section 2.
Note: Authorization
must be received prior to
biothreat agent transfer
Destroy all related
specimens, isolates,
and derivatives using
approved method.
Note: LRN Reference
Laboratory can
provide guidance
Biothreat Agent Response Algorithm
RESPONDING TO A BIOTHREAT AGENT
Sentinel Laboratory LRN Reference Laboratory
YES
YES
NO EXPOSURES
POTENTIAL EXPOSURES
NO
NO
OR
Notify Sentinel Laboratory

13
Gram Negative Bacilli/Coccobacilli Rule-Out Algorithm
BIOTHREAT AGENT IDENTIFICATION
Slow growing Gram negative bacilli/coccobacilli
Growth on MAC?
NO OR POOR GROWTH
Catalase positive?
Catalase positive?
Oxidase positive?
Urea positive?
Urea negative?
Indole negative?
Follow ASM
Brucella
guidelines
Oxidase negative?
Satellite negative?Satellite negative?
Gray, translucent,
non-hemolytic
colonies on BAP?
Grows better on
CHOC than BAP?
No hemolysis
on BAP?
Indole negative?
Follow ASM
Y. pestis
guidelines
Follow ASM
B. pseudomallei
guidelines
Follow ASM
B. mallei
guidelines
Follow ASM
F. tularensis
guidelines
Oxidase positive?
NO
NEGATIVE OR
WEAK POSITIVE
YES
YES
YES
YES
YES
YES
YESYES
YES
YES
YES VARIABLE
YES
YES
YES
YES

14
Handling Instructions
ANTHRAX — Bacillus anthracis
Safety
Patient specimens can be handled using BSL-2 practices.
As soon as B. anthracis is suspected, perform all further work within a Class II BSC using BSL-3 practices, especially when
performing activities with a high potential for aerosol or droplet production.
Potential Lab Exposures
Ingestion, inhalation, inoculation and direct contact via skin abrasions and mucous membranes.
Specimen Collection
Ideal Time & Temp
Transport
Within Facility
Storage
Cutaneous
Vesicular Stage
Collect uid from intact vesicles on sterile swab(s).
The organism is best demonstrated in this stage.
≤2 h RT ≤24 h RT
Eschar Stage
Without removing eschar, insert swab beneath the
edge of eschar, rotate and collect lesion material.
≤2 h RT ≤24 h RT
Gastrointestinal
Stool
Collect 5-10 g in a clean, sterile, leak proof container. ≤1 h RT ≤24 h 4°C
Blood
Collect per institution’s procedure for routine blood
cultures.
≤2 h RT
Incubate per
lab protocol
Inhalation
Sputum
Collect expectorated specimen into a sterile, leak proof
container.
≤2 h RT ≤24 h RT
Blood
Collect per institution’s procedure for routine blood
cultures.
≤2 h RT
Incubate per
lab protocol

15
Characterization
ANTHRAX — Bacillus anthracis
Gram Stain
• Large Gram positive rods
(1-1.5 µm x 3-5 µm)
• Direct smears of clinical specimens:
• Short chains (2-4 cells)
• Capsule present
• No spores present
• Smears from culture (BAP or CHOC):
• Long chains
• No capsule present
• Spores in older cultures: oval,
central to subterminal, no swelling
of cell wall
Biochemical/Test Reactions
• Catalase positive
• Non-motile
Colony Morphology
• Grows well on BAP and CHOC
• Aerobic rapid growth as early as 4-8h
• Colonies 2-5 mm on BAP and CHOC
at 24h
• No growth on MAC and EMB
• Flat or slightly convex with irregular
edges that may have comma-like
projections
• Ground-glass appearance
• Gamma hemolytic (non-hemolytic) on
BAP
• Tenacious, sticky colonies, adheres to
agar surface
Common Misidentifications
May not be identied in common
automated ID systems, including MALDI-
TOF, and possible misidentications
include Bacillus megaterium and other
Bacillus species.
Note: Bacillus cereus Group includes B. anthracis, but automated ID systems may not alert
microbiologist beyond this group identication.
Gram stain of blood culture
24h growth on BAP
Irregular-edged colonies

16
Rule-Out Algorithm
ANTHRAX — Bacillus anthracis
As soon as B. anthracis or B. cereus biovar anthracis is suspected perform all further work in a Class II BSC using BSL-3
practices. If B. anthracis or B. cereus biovar anthracis cannot be ruled out with the tests below, do not attempt further ID
using commercial automated or kit identication systems.
Gram stain morphology
□
Large, Gram positive rods?
Note: Spores may be found in cultures grown in 5% CO
2
or ambient
atmosphere but not usually observed in clinical samples.
Colony morphology
□
Ground glass appearance?
□
Non-pigmented, gamma hemolytic (no hemolysis) on BAP?
Note: Some strains of B. cereus biovar anthracis may be weakly
hemolytic after 48h
□
No growth on MAC (or EMB)?
Gamma hemolytic (no hemolysis)?
Bacillus anthracis
is ruled out
Bacillus anthracis and
B. cereus biovar anthracis
are ruled out
Catalase positive?
B. anthracis or B. cereus biovar anthracis not ruled-out. Do not attempt further identication and contact your LRN Reference
Level Laboratory to refer the isolate. Suggested Reporting Language: Possible Bacillus anthracis or B. cereus biovar anthracis
submitted to LRN Reference Level Laboratory for conrmatory testing.
YES TO ALL
NO
NO TO ANY
NO
YES
YES, STOP
Continue with routine
identication
SAFETY

17
Characterization
ANTHRAX — Bacillus cereus biovar anthracis
Characteristic B. anthracis B. cereus
B. cereus biovar anthracis
CI
1
CA
2
Hemolysis
3
—- + —- —-
Motility
4
—- + +/—-
Gamma phage susceptibility
5
+ —- —- —-
Penicillin G
6
S R S R
Capsule
7
+ Absent in vitro + +
24 h growth on BAP, 5% CO
2
of CI (left) and CA (right) strains
1
Côte d’Ivoire strains, from chimpanzees
2
Cameroon strains, from gorillas or chimpanzees
3
Hemolysis
+ ........beta hemolytic on sheep blood agar
– ........non-hemolytic
4
Motility
+ ........motile
– ........non-motile
+/– ...B. cereus biovar anthracis strains are
usually motile, including those recovered
from gorillas, chimpanzees, and
elephants. B. cereus biovar anthracis goat
strains from Democratic Republic of the
Congo were non-motile.
5
Gamma phage susceptibility
+ ........susceptible
– ........resistant
6
Penicillin G
S ........susceptible
R .......resistant
7
Capsule
+ ........Present

18
Recommendations
ANTHRAX — B. anthracis & B. cereus biovar anthracis
Sentinel-level laboratories should continue using the existing ASM Sentinel Level Clinical
Laboratory Guideline for B. anthracis to rule out or refer isolates of Bacillus spp. that
produce non-hemolytic colonies with a ground glass appearance and are non-motile. Until
new guidelines are available, the following recommendations should be considered:
1. Suspect Bacillus spp. isolates that are large, catalase positive, Gram positive rods,
and non-hemolytic at 24h incubation in ambient atmosphere or 5% CO
2
should be
tested for motility. Isolates can appear weakly hemolytic upon extended incubation
(48h) in ambient atmosphere and are more hemolytic in 5% CO
2
at 48h. Semi-solid
medium is recommended for motility to ensure consistent results.
2. Suspect isolates should be investigated to determine if the isolate is signicant
regardless of motility. If the isolate was recovered from a sterile site or from a wound
culture, follow the local public health guidelines to assess whether the public health
lab or clinical lab should contact the patient’s attending physician to determine
the likely clinical signicance (e.g., does the patient have an anthrax-like clinical
syndrome?). Appropriate travel history should be obtained as well. If the isolate is
deemed signicant, the local LRN reference laboratory should be contacted to obtain
guidance regarding the need to refer the isolate for conrmatory testing.

19
Handling Instructions
BRUCELLOSIS — Brucella spp.
Safety
Patient specimens can be handled using BSL-2 practices.
As soon as Brucella spp. is suspected, perform all further work within a Class II BSC using BSL-3 practices, especially when
performing activities with a high potential for aerosol or droplet production.
Potential Lab Exposures
Ingestion, inhalation, inoculation and direct contact via skin abrasions and mucous membranes. Brucella spp. have a very
low infectious dose and laboratory workers can acquire brucellosis from direct exposure to samples or cultures.
Specimen Collection
Ideal Time & Temp
Transport
Within Facility
Storage
Acute,
Subacute
or Chronic
Serum
Collect at least 1 mL acute phase specimen without anti-coagulant
as soon as possible after disease onset. Collect a second,
convalescent specimen 14-21 days after acute specimen collection.
~2 h RT -20°C
Blood
Collect per institution’s procedure for routine blood cultures.
Note: Slow-growing in automated blood culture systems, consider extended
incubations up to 2-3 weeks.
≤2 h RT
Incubate per
lab protocol
Bone
Marrow
Collect per institution’s surgical or pathology procedure. ≤15 min RT ≤24 h 4°C
Spleen
or Liver
Collect tissue samples at least the size of a pea. Submit in sterile
container. May add 1-2 drops of saline to keep moist.
≤1 h RT ≤24 h RT
20
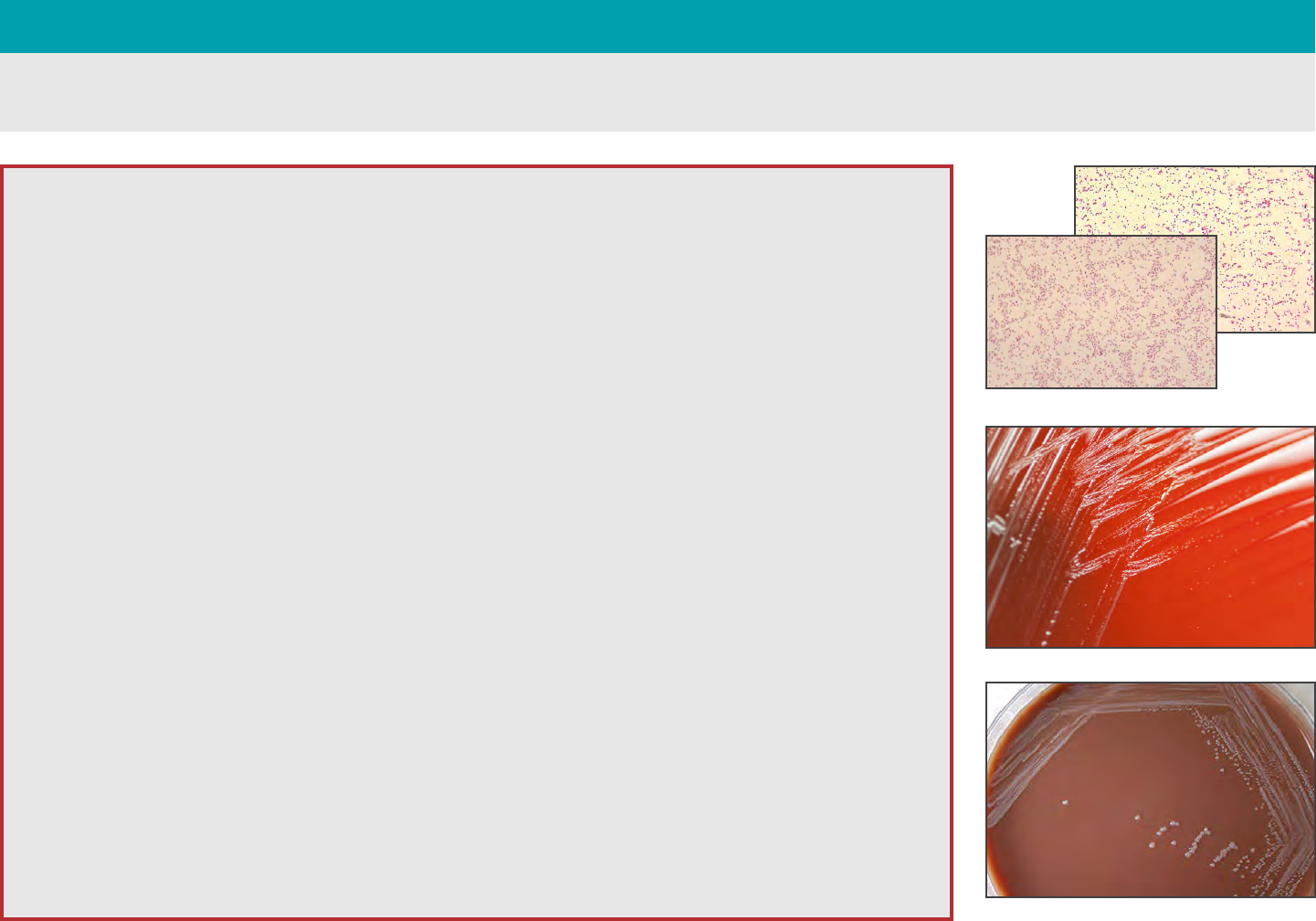
Characterization
BRUCELLOSIS — Brucella spp.
Gram Stain
• Faintly staining, not clustered, tiny
Gram negative coccobacilli
(0.4 µm-0.8 µm)
• May retain crystal violet stain and may
be mistaken for Gram positive cocci
Biochemical/Test Reactions
• Catalase, oxidase and urea positive
Note: Oxidase may be variable and test
should be performed on fresh cultures
(18-24h)
• S. aureus streak negative
(X & V Factor satellite test)
Colony Morphology
• Aerobic, slow growth
• Slow growth seen on BAP and CHOC
(CO
2
may be required by some strains)
• Poor to variable growth on MAC.
Pinpoint colonies may infrequently
be observed with some strains after
extended blood culture incubation
(7 days)
• Non-mucoid
• Pinpoint colonies at 24h, and easily
visible, discrete, white, non-hemolytic
colonies at 48h (0.5 mm-1 mm)
• Colonies on BAP have no
distinguishing features. They will
appear as white, non-pigmented and
non-hemolytic. Colonies will appear as
raised and convex with an entire edge
and shiny surface
Common Misidentifications
May not be identied in common
automated ID systems, including MALDI
TOF, and possible misidentications may
include: Moraxella spp., Micrococcus spp.,
Corynebacterium spp., “slow growing”
Staphylococcus spp., Oligella ureolytica,
Bordetella bronchiseptica, Haemophilus
spp., Pasteurella spp., Psychrobacter
phenylpyruvicus and Psychrobacter
immobilis.
Gram Stain
48h growth on BAP
72h growth on CHOC

21
Rule-Out Algorithm
BRUCELLOSIS — Brucella spp.
As soon as Brucella is suspected, perform all further work in a Class II BSC using BSL-3 practices. If Brucella spp. cannot
be ruled out with tests below, do not attempt further ID using commercial automated or kit identication systems.
Gram stain morphology
□
Faint staining, not clustered, tiny (0.4 x 0.8µm), Gram
negative coccobacilli?
Note: May retain crystal violet stain and be mistaken for Gram positive cocci
Growth
□
Subculture positive aerobic blood culture to BAP, CHOC?
□
Aerobic, slow, poorly growing colonies after 24h incubation in
5-10% CO
2
at 35°C?
Note: Incubate plates for at least two additional days if no growth in 24h.
□
Organism not growing on MAC?
□
Slow growing in automated blood culture systems?
Note: Consider extended incubations up to 2-3 weeks.
Consider Haemophilus
Consider Francisella
Refer to ASM sentinel procedures
Reincubate
Use internal laboratory procedure
48h growth on BAP
24h growth on CHOC
Brucella spp.
ruled out
YES
YES
YES, STOP
NO
NO
NO
Brucella spp. not ruled-out. Do not attempt further identication and contact your LRN
Reference Level Laboratory to refer the isolate. Suggested Reporting Language: Possible
Brucella spp. submitted to LRN Reference Level Laboratory for conrmatory testing.
Satellite negative at 24-48 hours?
Note: Spot BAP with S. aureus ATCC 25923
Oxidase and catalase positive?
Urea positive?
SAFETY
YES TO ALL
NO TO ANY

22
Handling Instructions
GLANDERS
—
Burkholderia mallei
&
MELIOIDOSIS
—
Burkholderia pseudomallei
Safety
Patient specimens can be handled using BSL-2 practices.
As soon as B. mallei or B. pseudomallei are suspected, perform all further work within a Class II BSC using BSL-3
practices, especially when performing activities with a high potential for aerosol or droplet production.
Potential Lab Exposures
Ingestion, inhalation, inoculation, and direct contact via skin abrasions and mucous membranes.
Specimen Collection
Ideal Time & Temp
Transport
Within Facility
Storage
Blood or
Bone Marrow
Collect using standard automated blood culture system per
institution’s procedure for routine blood culture.
≤2 h RT
Delayed entry
depends on
instrument
Sputum/Bronchial
Collect into sterile leak proof container. ≤2 h RT ≤24 h 4°C
Abscess Material
and Wounds
Tissue aspirate, tissue uid preferred to swab alternative. ≤2 h RT ≤24 h 4°C
Urine
Collect at least 1 mL in leak proof container. ≤2 h RT ≤24 h 4°C

23
Characterization
GLANDERS — Burkholderia mallei
Gram Stain
• Small straight or slightly curved Gram
negative coccobacilli (1.5 µm-3 µm x
0.5-1 µm) with rounded ends
• Cells arranged in pairs, parallel
bundles, or the Chinese letter form
Colony Morphology
• Aerobic
• On BAP:
• Pinpoint to small grey colonies at
24h that may become smooth,
grey, and translucent at 48h with
no distinctive odor
• Non-hemolytic
• On MAC: No growth or pinpoint
colorless colonies after 48h
• No pigment, even on Mueller Hinton
agar
• No growth at 42˚C
Biochemical/Test Reactions
• Catalase positive
• Oxidase variable; most are negative
• Spot indole negative
• Non-motile (Recommend tube test,
not wet mount, due to potential
aerosol production)
• Polymyxin B and colistin no zone,
penicillin resistant, amoxicillin-
clavulanate susceptible
Common Misidentifications
May not be identied in common
automated ID systems, including MALDI-
TOF, and possible misidentications
may include: Burkholderia cepacia,
Chromobacterium violaceum,
Pseudomonas stutzeri, Bacillus spp.,
Pandoraea spp., Ralstonia spp. other
nonfermenting Gram negative bacilli.
Note: B. pseudomallei and B. mallei are arginine positive, unlike other Burkholderia; the
arginine test may be in kit identication systems.
Gram Stain
48h growth on BAP
24h growth on BAP

24
Rule-Out Algorithm
GLANDERS — Burkholderia mallei
As soon as Burkholderia is suspected, perform all further work in a Class II BSC using BSL-3 practices. If B. mallei cannot
be ruled out with tests below, do not attempt further ID using commercial automated or kit identication systems.
Gram stain morphology
□
Small straight or slightly curved Gram
negative coccobacilli with rounded ends?
□
Cells arranged in pairs, parallel bundles
or the Chinese letter form?
Colony morphology
□
Poor growth at 24h on all media?
□
Better growth of grey, translucent
colonies without pigment or hemolysis
at 48h on BAP?
□
Poor or no growth on MAC in 48h?
□
No distinctive odor (from closed plate)?
Reactions
□
Oxidase-variable?
Consider
Brucella
B. mallei &
B. pseudomallei
ruled out
B. mallei
ruled out
Consider
B. pseudomallei
24h growth on BAP
48h growth on BAP
Burkholderia mallei not ruled-out. Do not attempt further identication and contact your LRN
Reference Level Laboratory to refer the isolate. Suggested Reporting Language: Possible
Burkholderia mallei submitted to LRN Reference Level Laboratory for conrmatory testing.
YES
YES
YES
NO
NO
NO
NO
Spot indole negative, catalase positive,
non-hemolytic on BAP, no pigment?
Polymyxin B or colistin: no zone; amoxicillin-
clavulanate susceptible; penicillin resistant?
No growth at 42°C and no odor?
Non-motile?
SAFETY
NO TO ANYYES TO ALL
YES, STOP

25
Characterization
MELIOIDOSIS — Burkholderia pseudomallei
Gram Stain
• Straight, or slightly curved Gram negative
rod (2-5 µm x 0.4-0.8 µm)
• Colonies may demonstrate bipolar
morphology in direct specimens and
peripheral staining in older cultures,
which can mimic endospores
Colony Morphology
• Aerobic
• On BAP: small, smooth, creamy colonies
in the rst 1-2 days, that may gradually
change in time to dry, wrinkled colonies
(similar to Pseudomonas stutzeri)
• Poor growth at 24h, good growth at 48h
• Colonies are non-hemolytic and not
pigmented on BAP or Mueller Hinton agar.
• Grows on MAC (may uptake pink dye)
• Distinctive musty, earthy odor is apparent
without snifng or opening plate
• Growth at 42˚C
Biochemical/Test Reactions
• Oxidase positive
• Spot indole negative
• Motile
Note: Tube test, not wet mount, is
recommended due to potential aerosolization
• Polymyxin B and colistin no zone,
penicillin resistant, amoxicillin-clavulanate
susceptible
Common Misidentifications
May not be identied in common automated
ID systems, including MALDI TOF, and
possible misidentications may include:
Burkholderia cepacia*, Chromobacterium
violaceum, Pseudomonas aeruginosa,
Pseudomonas stutzeri, S. maltophilia and
other nonfermenting Gram negative bacilli.
* B. pseudomallei is separated from B. cepacia by a
susceptible amoxicillin-clavulanate test. Although rare
in B. pseudomallei, resistance cannot rule out the
identication.
Note: B. pseudomallei and B. mallei are arginine positive, unlike other Burkholderia; arginine test may be
in kit identication systems. Also, unlike B. mallei, B. pseudomallei grows at 42°C in 48h and is motile.
Gram Stain
24h growth on BAP
48h growth on BAP
48h growth on MAC

26
Rule-Out Algorithm
MELIOIDOSIS — Burkholderia pseudomallei
As soon as Burkholderia is suspected, perform all further work in a Class II BSC using BSL-3 practices. If B. pseudomallei
cannot be ruled out with tests below, do not attempt further ID using commercial automated or kit identication systems.
24h growth on BAP
48h growth on BAP
Gram stain morphology
□
Gram negative rod, straight or slightly
curved?
Note: May demonstrate bipolar morphology
at 24h and peripheral staining, like
endospores, as cultures age.
Colony morphology
□
Poor growth at 24h, but good growth of
smooth, creamy colonies at 48h on BAP?
Note: May develop wrinkled colonies in
time
□
Non-hemolytic?
□
Strong musty/earthy odor (apparent without
opening plate), growth on MAC in 48h?
□
Non-pigmented on Mueller Hinton agar
and BAP?
Reactions
□
Oxidase positive, spot indole negative?
Growth on MAC?
Oxidase positive and
spot indole negative?
Polymyxin B or colistin; no zone or
growth on B. cepacia selective agars
No hemolysis on BAP; not pigmented
Burkholderia pseudomallei not ruled-out. Do not attempt further identication and contact your
LRN Reference Level Laboratory to refer the isolate. Suggested Reporting Language: Possible
Burkholderia pseudomallei submitted to LRN Reference Level Laboratory for conrmatory testing.
Not Burkholderia
Rule out other agents such as
B. mallei, Brucella and Francisella
Not Burkholderia
Consider Chromobacterium violaceum
or indole-negative Vibrio spp.
YES
YES
YES
NO
NO
NO
NO
SAFETY
YES TO ALL NO TO ANY
YES, STOP

27
Handling Instructions
TULAREMIA — Francisella tularensis
Safety
Patient specimens can be handled using BSL-2 practices.
As soon as F. tularensis is suspected, perform all further work within a Class II BSC using BSL-3 practices, especially when
performing activities with a high potential for aerosol or droplet production.
Potential Lab Exposures
Ingestion, inhalation, inoculation, and direct contact via skin abrasions and mucous membranes. Francisella tularensis has
a very low infectious dose and laboratory workers can acquire Tularemia from direct exposure to samples or cultures.
Specimen Collection
Ideal Time & Temp
Transport
Within Facility
Storage
Sputum or
Throat
Collect routine throat culture using a swab or expectorated sputum collected into a
sterile, leak proof container.
≤2 h RT ≤24 h 4°C
Bronchial or
Tracheal Wash
Collect per institution’s procedure in an area dedicated to collecting respiratory specimens
under isolation or containment circumstances (i.e., isolation chamber or “bubble”).
≤2 h RT ≤24 h 4°C
Blood
Collect per institution’s procedure for routine blood cultures. ≤2 h RT
Incubate per
lab protocol
Biopsy, Tissue,
Scrapings,
Aspirate
or Swab
Submit in sterile container. For small tissue samples add several drops of sterile
normal saline to keep tissue moist. For swabs, collect by obtaining rm sample of
advancing margin of the lesion; place swab in transport package to keep moist with
the transport medium inside packet.
≤2 h RT ≤24 h 4°C
Serum
Collect at least 1 mL without anticoagulant. Collect acute specimen as soon as
possible after onset and a convalescent specimen >14 days after acute.
≤2 h RT 4°C

28
Characterization
TULAREMIA — Francisella tularensis
Gram Stain
• Tiny, Gram negative coccobacilli
(0.2-0.5 µm x 0.7-1.0 µm)
• Poor counterstaining with safranin
(basic fuchsin counterstain may
increase resolution)
• Pleomorphic
• Mostly single cells
Colony Morphology
• Aerobic, fastidious
• No growth on MAC or EMB
• Scant or no growth on BAP; may
grow on primary culture, not well on
subculture
• Slow growing on CHOC, TM or BCYE:
1-2 mm after 48h
• Colonies are opaque, grey-white,
butyrous with smooth and shiny
surface
Biochemical/Test Reactions
• Oxidase negative
• Catalase negative or weakly positive
• Satellite negative
• Beta-lactamase positive
Common Misidentifications
May not be identied in common
automated ID systems, including MALDI
TOF, and possible misidentications
may include: Aggregatibacter
actinomycetemcomitans, Haemophilus
inuenzae, Oligella spp. and
Psychrobacter spp.
Gram Stain
Gram stain of a blood culture
48h growth on CHOC

29
Rule-Out Algorithm
TULAREMIA — Francisella tularensis
As soon as Francisella is suspected, perform all further work in a Class II BSC using BSL-3 practices. If F. tularensis cannot
be ruled out with tests below, do not attempt further ID using commercial automated or kit identication systems.
Gram stain morphology
□
Pleomorphic?
□
0.2–0.5 µm by 0.7–1.0 µm faintly
staining, Gram negative coccobacillus?
□
Mostly single cells?
Colony morphology
□
Aerobic and fastidious?
□
No growth on MAC/EMB
□
Scant to no growth on BAP after 48h?
Note: may grow on primary BAP culture, but
not on subculture.
□
Slow growth on CHOC, TM or BCYE?
□
1-2 mm gray to grayish-white colonies
on CHOC after 48h
□
Colonies opaque, grey-white, butyrous
with smooth and shiny surface?
24h: Growth on CHOC but not BAP?
48h: Growth better on CHOC than BAP?
Are colonies satellite negative?
Oxidase negative and either
catalase weakly positive or negative?
β-lactamase positive?
No growth on MAC?
Francisella tularensis not ruled-out. Do not attempt further identication and contact
your LRN Reference Level Laboratory to refer the isolate. Suggested Reporting Language:
Possible F. tularensis submitted to LRN Reference Level Laboratory for conrmatory testing.
Consider
Haemophilus
Francisella tularensis ruled out.
Continue with routine identication
48h growth on BAP
48h growth on CHOC
YES
YES
YES
YES
NO
NO
NO
NO
NO
SAFETY
NO TO ANYYES TO ALL
YES, STOP

30
Handling Instructions
PLAGUE — Yersinia pestis
Safety
Patient specimens can be handled using BSL-2 practices.
As soon as Y. pestis is suspected, perform all further work within a Class II BSC using BSL-3 practices, especially when
performing activities with a high potential for aerosol or droplet production.
Potential Lab Exposures
Ingestion, inhalation, inoculation, and direct contact via skin abrasions and mucous membranes.
Specimen Selection
Ideal Time & Temp
Transport
Within Facility
Storage
Pneumonic
Sputum or
Throat
Collect routine throat culture using a swab or expectorated
sputum collected into a sterile, leak proof container
≤2 h RT ≤24 h 4°C
Bronchial
or Tracheal
Wash
Collect per institution’s procedure in an area dedicated to
collecting respiratory specimens under isolation or containment
circumstances (i.e., isolation chamber or “bubble”)
≤2 h RT ≤24 h 4°C
Septicemic Blood
Collect per institution’s procedure for routine blood cultures ≤2 h RT
Incubate per
lab protocol
Bubonic
Tissue or
Aspirate
Submit in sterile container, may add 1-2 drops of saline to keep
moist
≤2 h RT ≤24 h 4°C

31
Characterization
PLAGUE — Yersinia pestis
Gram Stain
• Plump Gram negative rods
(0.5 x 1-2 µm) seen mostly as single
cells or pairs, and may demonstrate
short chains in liquid media
• May exhibit bipolar, “safety-pin”
appearance that is not seen on
Gram stain, may be exhibited by
Giemsa stain or Wright's stain
Colony Morphology
• Facultative anaerobe
• Slow growing at 35˚C, better growth
at 25-28˚C
• Grey-white, translucent pinpoint
colonies at 24h, usually too small to
be seen
• On BAP:
• After 48h: colonies approximately
1-2 mm in diameter, gray-white to
slightly yellow and opaque
• Older cultures (~96h): “Fried
egg” or “hammered copper”
appearance (under magnication)
• Little to no hemolysis
• Lactose non-fermenter at 48h on
MAC or EMB
Biochemical/Test Reactions
• Catalase positive
• Oxidase, urease (at 35˚C) and indole
negative
Common Misidentifications
May not be identied in common automated
ID systems, including MALDI TOF, and
possible misidentications may include:
Shigella spp., H
2
S(-) Salmonella spp.,
Acinetobacter or Pseudomonas spp. and
Yersinia pseudotuberculosis.
Gram Stain
48h growth on BAP
24h growth on BAP at 25°C (left) and 35°C (right)

32
Rule-Out Algorithm
PLAGUE — Yersinia pestis
48h growth on MAC
Fried egg appearance at 96h
(magnified)
As soon as Yersinia is suspected, perform all further work in a Class II BSC using BSL-3 practices. If Y. pestis cannot be
ruled out with tests below, do not attempt further ID using commercial automated or kit identication systems.
Gram stain morphology
□
Gram-negative plump rods,
0.5 x 1-2 µm?
Note: Seen mostly as single cells or pairs,
and may demonstrate short chains in liquid
media.
Colony morphology
□
Facultative anaerobe?
□
Slow growing at 35°C with better growth
at 25-28°C?
□
Either pinpoint colonies or no growth on
BAP after 24h
□
Colonies are 1-2 mm, gray-white to
slightly yellow and opaque on BAP after
48h?
□
Non-lactose fermenter on MAC/EMB?
□
“Fried egg” or “hammered copper” on
BAP in older cultures (~96h), when
magnied?
□
Little to no hemolysis on BAP?
Oxidase negative, catalase positive
and indole negative?
Yersinia pestis not ruled-out. Do not attempt further identication and contact your LRN
Reference Level Laboratory to refer the isolate. Suggested Reporting Language:
Possible Y. pestis submitted to LRN Reference Level Laboratory for conrmatory testing.
Yersinia pestis ruled out.
Continue routine identication
YES
YES
NO
Urease negative at 35°C?
SAFETY
YES TO ALL
NO TO ANY
YES, STOP
33
APHL ..............Association of Public Health
Laboratories
ASM ...............American Society for Microbiology
BAP .................Blood agar plate
BCYE ...............Buffered Charcoal Yeast Extract
BSC .................Biological safety cabinet
BSL ..................Biosafety Level (1 - 4)
BT .....................Biothreat
CDC .................Centers for Disease Control and
Prevention
CHOC ..............Chocolate agar
EMB .................Eosin Methylene Blue agar
LRN .................Laboratory Response Network
MAC ...............MacConkey agar
MALDI TOF .... Matrix Assisted Laser Desorption/
Ionization Time of Flight Mass
Spectrometer
NF ....................Non-fermentor
PPE ..................Personal Protective Equipment
RT ....................Room Temperature
TM ....................Thayer Martin agar
TTC...................2,3,5-Triphenyltetrazolium
chloride
Acronyms
APPENDIX

34
Administrative controls
Changes in work procedures such as written
safety policies, work practices, rules, supervision,
schedules and training with the goal of reducing
the duration, frequency and severity of exposures
to hazardous materials or situations.
Aerobic
Requiring oxygen.
Aerosolization
The generation of liquid droplets or particles, ve
microns or less in diameter, that can be inhaled
and retained in the lungs.
Anaerobic
Requiring the absence of oxygen.
Antimicrobial
An agent that kills microorganisms or suppresses
their growth and multiplication.
Antiseptic
A substance that inhibits the growth and
development of microorganisms without
necessarily killing them. Antiseptics are usually
applied to body surfaces.
Barriers
Any method used to separate workers, the outside
community and the environment from hazardous
material; includes primary and secondary barriers.
Barriers, Primary
Specialized laboratory equipment with
engineering controls designed to protect against
exposure to hazardous laboratory materials,
including, but not limited to, biologic safety
cabinets, chemical fume hoods, enclosed
containers, bench shields, animal cages, and
engineered sharps injury-protection devices
(e.g., safety needles, safety scalpels, and sharps
containers).
Barriers, Secondary
Facility design and construction features to
include, but not be limited to, directional air
ow, entrance airlocks, controlled-access
zones, HEPA-ltered exhaust air, facility controls,
decontamination equipment, eyewash stations,
protective showers, and sinks for hand washing.
Biohazardous materials
Infectious agents or hazardous biologic materials
that present a risk or potential risk to the health
of humans, animals, or the environment. The risk
can be direct through infection or indirect through
damage to the environment. Biohazardous
materials include certain types of recombinant
DNA, organisms and viruses infectious to humans,
animals, or plants (e.g., parasites, viruses,
bacteria, fungi, prions, and rickettsia), and
biologically active agents (e.g., toxins, allergens,
and venoms) that can cause disease in other
living organisms or cause signicant impact to the
environment or community.
BSL-1
Biosafety Level 1 is suitable for work involving
well-characterized agents not known to
consistently cause disease in immunocompetent
adult humans, and present minimal potential
hazard to laboratory personnel and the
environment.
BSL-2
Biosafety Level 2 builds upon BSL-1. BSL-
2 is suitable for work involving agents that
pose moderate hazards to personnel and the
environment. (Most Sentinel Laboratory facilities
fall under the denition of BSL-2).
BSL-3
Biosafety Level 3 is applicable to clinical,
diagnostic, teaching, research, or production
facilities where work is performed with indigenous
or exotic agents that may cause serious or
potentially lethal disease through the inhalation
route of exposure.
Terms and Definitions
APPENDIX

35
BSL-4
Biosafety Level 4 is required for work with
dangerous and exotic agents that pose a high
individual risk of aerosol-transmitted laboratory
infections and life-threatening disease that is
frequently fatal, for which there are no vaccines or
treatments, or a related agent with unknown risk
of transmission.
Containment
Methods used to shield or protect personnel, the
immediate work environment, and the community
from exposure to hazardous, radiologic, chemical,
or biologic materials.
Decontamination
The removing of chemical, biologic, or radiologic
contamination from, or the neutralizing of it on, a
person, object, or area. Any process for removing
and/or killing microorganisms. The same term is
also used for removing or neutralizing hazardous
chemicals and radioactive materials.
Disinfectant
A chemical or mixture of chemicals used to kill
microorganisms, but not necessarily spores.
Disinfectants are usually applied to inanimate
surfaces or objects.
Disinfection
A physical or chemical process of reducing or
eliminating microorganisms from a surface or
space, but not necessarily spores.
Droplet nuclei
The residue of dried droplets of infectious agents
that is easily inhaled and exhaled and can remain
suspended in air for relatively long periods or be
blown over great distances.
Droplet spread
The direct transmission of an infectious agent
by means of the aerosols produced in sneezing,
coughing, or talking that travel only a short
distance before falling to the ground.
Engineering controls
Refers to methods to remove a hazard or place
a protective barrier between the worker and the
workplace hazard, which usually involves building
design elements and specialized equipment.
Exposure
Having come into contact with a cause of, or
possessing a characteristic that is a determinant
of, a particular health problem.
Fomite
An inanimate object that can be the vehicle for
transmission of an infectious agent (e.g., bedding,
towels or surgical instruments).
Incident
An unexpected event that causes or has the
potential to cause loss, injury, illness, unsafe
conditions, or disruptions to normal procedures.
Incubation period
The time interval from exposure to an infectious
agent to the onset of symptoms of an infectious
disease.
Infection
Invasion of the body tissues of a host by an
infectious agent, whether or not it causes disease.
Medical surveillance
Monitoring of a person who might have been
exposed to an infectious, chemical, radiologic, or
other potentially causal agent, for the purpose of
detecting early symptoms.
Mitigate
To correct identied deciencies and to make
a hazard less severe. This includes corrective
actions taken as a result of an inspection or audit,
or after an incident.
Mode of transmission
The manner in which an agent is transmitted from
its reservoir to a susceptible host.
Terms and Definitions
APPENDIX

36
Personal protective equipment (PPE)
Items worn by laboratory workers to prevent direct
exposure to hazardous materials, including gloves,
gowns, aprons, coats, containment suits, shoe
covers, eye and face shields, respirators, and masks.
Risk
The probability that an event will occur (e.g., that
a person will be affected by, or die from, an
illness, injury, or other health condition within a
specied time or age span).
Risk assessment
A process to evaluate the probability and
consequences of exposure to a given hazard, with
the intent to reduce the risk by establishing the
appropriate hazard controls to be used.
Risk factor
An aspect of personal behavior or lifestyle,
an environmental exposure, or a hereditary
characteristic that is associated with an increase
in the occurrence of a particular disease, injury,
or other health condition.
Routes of exposure
Paths by which humans or other living organisms
come into contact with a hazardous substance.
Three routes of exposure are breathing
(inhalation), eating or drinking (ingestion), and
contact with skin (dermal absorption).
Sharps
Items capable of cutting or piercing human skin.
Examples include hypodermic needles, syringes
(with or without attached needles), Pasteur
pipettes, scalpel blades, suture needles, blood
vials, needles with attached tubing, and culture
dishes (regardless of presence of infectious
agents). Also included are other types of broken
or unbroken glassware that have been in contact
with infectious agents (e.g., used microscope
slides and cover slips).
Sterilization
The use of physical or chemical process to
completely destroy or eliminate all classes of
microorganisms and spores.
Symptom
Any indication of disease noticed or felt by a
patient.
Transmission (of infection)
Any mode or mechanism by which an infectious
agent is spread to a susceptible host. Airborne
transmission is the transfer of an agent
suspended in the air (considered a type of
indirect transmission). Direct transmission
is the immediate transfer of an agent from a
reservoir to a host by direct contact or droplet
spread. Indirect transmission is the transfer of an
agent from a reservoir to a host either by being
suspended in air particles (airborne), carried by
an inanimate objects (vehicleborne), or carried by
an animate intermediary (vectorborne).
TTC
2,3,5-Triphenyltetrazolium chloride, indicator dye
within motility test medium.
Universal precautions
Guidelines recommended by CDC for reducing
the risk for transmission of bloodborne and
other pathogens in hospitals, laboratories, and
other institutions in which workers are potentially
exposed to human blood and body uids. The
precautions are designed to reduce the risk
for transmission of microorganisms from both
recognized and unrecognized sources of infection
in hospitals, laboratories, and other institutions to
the workers in these facilities.
Virulence
The ability of an infectious agent to cause severe
disease, measured as the proportion of persons
with the disease who become severely ill or die.
Zoonosis
An infectious disease that is transmissible from
animals to humans.
Terms and Definitions
APPENDIX

37
Urea
Look for pink color change
Negative Positive
Identification Tests
APPENDIX
Spot Indole
Look for color change, varies by
reagent; Cinnamaldehyde preferred
Cinnamaldehyde:
positive is blue
Benzaldehyde:
positive is pink
Arginine Dihydrolase (Decarboxylase)
Look for pink/purple color change
Uninoculated
Base
Positive Base Negative Positive
Controls
NF Base Positive
Catalase
3% Hydrogen peroxide: look for bubbles
Negative Weak Positive Positive
Safety Note: Recommended to perform this test in a
BSC, covered petri dish or tube to contain aerosols
Oxidase
Tetramethyl reagent: look for purple color change
Negative Positive

38
X/V Factor Satellite Test
Use Staphylococcus aureus-streaked media
or X and V growth factor-impregnated discs
Negative
Growth is not isolated to area immediately
adjacent to S. aureus streak or X and V factors
Positive (Satellite)
Growth occurs only along S. aureus streak/
X and V factors
Motility
Negative (Non-motile)
Growth only in line of inoculum; no fuzziness or
spreading; media is clear
Intermediate
Start to see growth outside line of inoculum
(appears fuzzy), media still clear
Positive (Motile)
Distinct growth outside line of inoculum into the
media, which is not clear
Safety Note: Avoid wet mount motility tests, which are hazardous
due to the potential for creating an aerosol. Perform a tube motility
test instead, and always in a BSC.
Identification Tests
APPENDIX
Negative: Brucella growing across entire plate
Positive: Haemophilus growing only around
the Staphylococcus aureus streak
Negative Intermediate Positive
With 2,3,5-Triphenyltetrazolium chloride (TTC)
TTC: Colorless medium dye, turns red when reduced by
bacteria. Inhibits some bacteria; look for growth away
from line of inoculum.
Negative Positive
No Additives

39
APHL
Public Health Preparedness & Response Program
aphl.org/programs/preparedness/Pages/default.aspx
Lab Biosafety & Biosecurity Resources
aphl.org/programs/preparedness/Pages/Strengthening-Lab-Biosafety-
Biosecurity.aspx
State Public Health Laboratories Emergency Contact
Directory
aphl.org/programs/preparedness/Crisis-Management/Pages/
Emergency-Lab-Contacts.aspx
Training Department
aphl.org/training/Pages/default.aspx
ASM
Sentinel Level Clinical Laboratory Protocols for Suspected
Biological Threat Agents and Emerging Infectious Diseases
(Includes sentinel laboratory definition & emergency contacts)
asm.org/Articles/Policy/Laboratory-Response-Network-LRN-Sentinel-
Level-C
CDC
Biosaf
ety in Microbiological and Biomedical Laboratories
(5th Edition)
cdc.gov/biosafety/publications/bmbl5/
Federal Select Agent Program
selectagents.gov
Federal Select Agent Program Forms
selectagents.gov/forms.html
Morbidity and Mortality Weekly Report,“Guidelines for
Safe Work Practices in Human and Animal Medical
Diagnostic Laboratories.”
cdc.gov/mmwr/preview/mmwrhtml/su6101a1.htm
CDC TRAIN
train.org/cdctrain/welcome
New York State Dept. of Health, Wadsworth Center
Basic Select Agent Flow Chart & Evaluation (B-SAFE) Bench Cards
• health.ny.gov/guidance/oph/wadsworth/nal_card.pdf
• health.ny.gov/guidance/oph/wadsworth/
State Hygienic Laboratory at the University of Iowa
Education/Training Resources
shl.uiowa.edu/edtrain/index.xml
Resources
APPENDIX

Association of Public Health Laboratories
The Association of Public Health Laboratories (APHL) works to strengthen laboratory
systems serving the public’s health in the US and globally. APHL’s member laboratories
protect the public’s health by monitoring and detecting infectious and foodborne
diseases, environmental contaminants, terrorist agents, genetic disorders in newborns
and other diverse health threats.
8515 Georgia Avenue, Suite 700
Silver Spring, MD 20910
Phone: 240.485.2745
Fax: 240.485.2700
Web: www.aphl.org
© Copyright 2017, Association of Public Health Laboratories. All Rights Reserved.
